For years we’ve been transfusing red cells in the ED to patients who don’t actually need them. A study looking at trends in transfusion practice in the ED found that about 1/3 of transfusions given were deemed totally inappropriate. As we explained in previous EM Cases episodes, there have been a whole slew of articles in the literature over the years that have shown that morbidity and mortality outcomes with lower hemoglobin thresholds, like 70g/L for transfusing ICU patients (TRICC trial), patients in septic shock (TRISS trial), and patients with GI bleeds are similar to outcomes with traditional higher hemoglobin thresholds of 90 or 100g/L. We’re simply transfusing blood way too much! The American Association of Blood Banks in conjunction with the American Board of Internal Medicine’s Choosing Wisely campaign, as one of its 5 statements on overuse of procedures, stated, “don’t transfuse iron deficiency without hemodynamic instability”.
So, in this episode with the help of Transfusion specialist, researcher and co-author of the American Association of Blood Banks transfusion guidelines Dr. Jeannie Callum, Transfusion specialist and researcher Dr. Yulia Lin, and ‘the walking encyclopedia of EM’ Dr. Walter Himmel, we give you an understanding of why it’s important to avoid red cell transfusions in certain situations, why IV iron is sometimes a better option in a significant subset of anemic patients in the ED, and the practicalities of exactly how to administer IV iron.
Written Summary and blog post prepared and written by Dr. Michael Kilian, edited by Dr. Anton Helman, May 2015
Cite this podcast as: Callum, J, Lin, Y, Himmel, W, Helman, A. IV Iron for Anemia in Emergency Medicine. Emergency Medicine Cases. May, 2015. https://emergencymedicinecases.com/iv-iron-for-anemia-in-emergency-medicine/. Accessed [date].
Menorrhagia and IV Iron for Anemia in Emergency Medicine
Case 1
ID: 49 year old woman sent in by her family physician with a note indicating “severe menorrhagia for several months and hemoglobin 57 g/L; please transfuse”.
PMH: Nil
HPI: Decreased exercise tolerance with increasingly heavy periods for several months. She denies dizziness or syncope.
O/E: Vitals and exam all within normal limits
How would you manage this patient’s anemia?
Is severe anemia unsafe in healthy people?
In a study of healthy subjects from JAMA in 1998 entitled ‘Human Cardiovascular and Metabolic Response to Acute Severe Isovolemic Anemia’, in which aliquots of blood (450-900 mL) were removed to reduce blood hemoglobin concentration from 131g/L to 50 g/L and isovolemia was maintained with 5% human albumin and/or autologous plasma, they found that acute isovolemic reduction of blood hemoglobin concentration to 50 g/L did not produce evidence of inadequate systemic “critical” oxygen delivery, as assessed by lack of change of O2 and plasma lactate concentration. Analysis of Holter readings suggested that at this hemoglobin concentration in this resting healthy population, myocardial ischemia would occur infrequently.
Compensation in chronic vs acute anemia
Patients with chronic anemia can adjust physiologically to anemia even more readily than patients with acute anemia because of the shift in the oxygen dissociation curve. This is facilitated by a change in the 2,3-DPG level allowing the RBCs to be ‘less selfish’ so they can more easily offload oxygen to the tissues. As such, a hemoglobin of 50g/L can be considered as physiologically higher than it appears in patients with chronic anemia.
The WOMB Trial showed that young women can safely tolerate a hemoglobin as low as 50g/L
The WOMB trial was a multi-centered Dutch trial that enrolled 521 women with severe postpartum anemia (hemoglobin 48 to 79 g/L) who were randomized to transfusion or transfusion only if they developed severe symptoms. It found no differences in any important outcomes (recovery of hemoglobin, 6 week hemoglobin). There was, however, a non-clinically significant difference in fatigue scores at 7 days that was not persistent at later time point. 517 units were transfused to the “transfused group” vs. 88 for the group for only severe symptoms.
Allo-immunization is the most important and under-recognized risk associated with red cell transfusions
Perhaps the most important and under-recognized risk of red cell transfusions is allo-immunization among women of childbearing age.
Allo-imunization, which has a rate of 8% per transfusion in young women, involves the development of antibodies against red blood cells, which in future pregnancies can cross the placenta and precipitate hemolytic disease of the newborn in women who have received previous transfusion, can render the patient ineligible for an organ transplant if required and can make them unmatchable for future transfusions.
Think of a blood transfusion as a blood transplant: When you give someone a blood transfusion, you are changing their immune system for life. Red cell transfusions should not be thought of as a delivery system for iron!
Other risks of packed red blood cell transfusions include a 1/700 risk of TACO (Transfusion Associated Circulatory Overload) a 1/10,000 risk of TRALI (Transfusion Related Lung Injury) and a 1/40,000 risk of an acute hemolytic transfusion reaction.
Indications for Iron for anemia in Emergency Medicine
The American Society of Anesthesiologists recommend against RBC transfusions in young, healthy patients without ongoing blood loss and a hemoglobin >60 g/L, unless they are symptomatic or hemodynamically unstable. Symptoms to screen for include chest pain, SOB, pre-syncope, lightheadedness, hypotension and tachycardia.
Fatigue, pallor and reduced exercise tolerance are NOT, in and of themselves, an indication for red cell transfusion.
The trigger for transfusion related to “ongoing blood loss” will depend on acuity of blood loss, volume of ongoing bleeding and hemodynamic instability.
Indications for IV Iron:
- Oral iron poorly tolerated or failure of oral trial
- Poor oral absorption (ie. gastric bypass, celiac disease, gastritis)
- Rate of bleeding too brisk for oral iron
- Severe anemia (Hb <90g/L) especially if ongoing bleeding
- Time-sensitive pressures (OR etc.)
The main contraindications to IV Iron are active systemic infection (eg: suspected sepsis) since iron is a good microbial nutrient, and a known allergic or hypotensive reaction in the past.
Risks of IV Iron
- Hypotension (1-2%)
- Serious allergic reactions (< 1 in 1,000,000)
Other more common adverse reactions include joint aches, muscle cramps, headache, chest discomfort, nausea, vomiting and diarrhea, which generally resolve spontaneously within 24hrs of administration of IV iron.
Update 2015: In patients with chronic kidney disease IV iron may result in more infections and cardiovascular complications than oral iron. Full article
Update 2018: A quality improvement initiative aimed at improving the rate of appropriate transfusions for iron deficiency anemia (i.e. pRBC, IV iron) in the emergency department, demonstrated the use of educational presentations, algorithm development and toolkit creation led to an increase in RBC transfusion appropriateness from 53% to 91%. This improvement was achieved and maintained using simple and practical interventions over the course of 2 years. Abstract
Administering IV Iron for anemia in Emergency Medicine
The product you choose will depend on the dose you want to give, how quickly you want to deliver it and the side-effect profile (see order set example at end of summary)
| Iron Sucrose (Venofer) | Ferumoxytol (Feraheme) | |
| Dose (Max) | 300mg in 250mL NS | 510mg in 17mL (add to 50ml NS) |
| Infusion Time | 2 hrs | 15-60 mins |
| Serious Hypersensitivity | 0.6 per million | |
| Cost | $120 | $200 |
Patients with the following risk factors should receive slower infusions (e.g. Feraheme® [ferumoxytol] over 60 minutes or Venofer® [iron sucrose] 300mg over 2 hours)
- Age > 65 yrs
- Baseline systolic BP less than 100
- Severe asthma or eczema
- Severe respiratory or cardiac disease
- Treatment with beta-blockers, ACE inhibitors or 3 or more anti-hypertensive medications
- Nephrology patients
After IV Iron, and with ongoing oral supplementation, a patient’s hemoglobin will start to rise 3-7 days after the IV infusion. You can expect a 1-2 point rise in the hemoglobin per day, and after 2-4 weeks the hemoglobin will have risen 20-30g/L.
Update 2016: Faraheme is no longer available in Canada or the EU
Sunnybrook Hospital IV Iron Indications & Criteria for use (with permission)
Sunnybrook Hospital IV Iron order sheet (with permission)
Oral Iron supplementation after IV Iron:
Ferrous sulfate 300mg 1 tab QHS: contains 60mg of elemental iron
- Take at bedtime on empty stomach at least 2 hours after meals with Vitamin C 500mg
- Avoid taking with calcium or magnesium supplements as these decrease absorption.
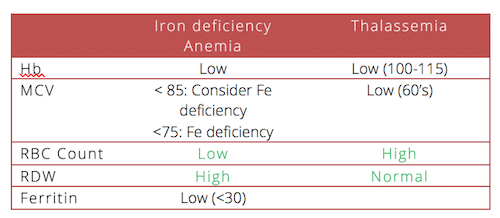
Lab Interpretation to determine iron deficiency
Patients with thalassemia may also have iron deficiency contributing to their anemia. If a patient with thalassemia has a Ferritin <30, consider them iron deficient.
IV Iron instead of recurrent red cell transfusions for anemia of chronic disease
Case 2:
ID: 85 year old male from nursing home sent in for “usual red cell transfusion” which he receives on a monthly basis.
HPI: Patient denies chest pain, shortness of breath, palpitations, dizziness or melena
PMH: CHF, CRF
Hb= 65 g/L
Would you transfuse this patient with RBCs?
Most of the patients in the ED found to be anemic are elderly. These patients generally fall into one of three categories: One third will have a simple nutritional deficiency (iron or B12). One third will have anemia secondary to a chronic disease. The rest of the patients will have an undifferentiated cause of their anemia that will require further investigation.
For an elderly patient with multiple comorbidities it can be challenging to determine if their anemia is secondary to iron deficiency as well as anemia of chronic disease. To you help differentiate and decide whether a patient would benefit from IV iron and supplementation, our experts have suggested the following approach:
Which pre-operative patients require red cell transfusions?
Case 3:
ID: 82 year old woman with a mechanical fall at home. She was unable to stand up and called EMS, who noted an externally rotated and shortened right leg.
PMH: Diabetes, hypothyroidism, hypertension, hypercholesterolemia, B12 deficiency anemia (non compliant with treatment)
HPI: Has had fatigue and SOBOE which has been unchanged for months. She denies chest pain.
O/E: Vitals within normal limits, no orthostatic changes.
Initial blood work reveals a Hb= 83 g/L in ED.
After speaking with the orthopedic surgeon on call, they request that you transfuse 2 units of pRBCs in preparation for the OR.
Do you order a pRBC transfusion for this patient?
IV Iron for anemia in pre-operative patients?
The FOCUS trial sought to determine whether a higher threshold for blood transfusion would improve recovery in patients who had undergone surgery for a hip fracture. They showed that even among elderly patients with known coronary artery disease or multiple coronary risk factors, there was less mortality post-operatively at 30 and 90 days among patients with a transfusion trigger of 80 g/L compared to those with a higher transfusion trigger.
Observational studies of the use IV iron pre-operatively for patients with anemia have shown a reduced rate of red cell transfusion required.
Patient hand-out for iron therapy from Sunnybrook Hospital (with permission)
For more information on anemia on EM Cases:
New Rapid Reviews Videos on IV Iron & Hyponatremia
Key References
Weiskopf, R et al.,Human Cardiovascular and Metabolic Response to Acute, Severe Isovolemic Anemia.JAMA. 1998;279(3):217-221. Abstract
Litton E, Xiao J, Ho KM. Safety and efficacy of intravenous iron therapy in reducing requirement for allogeneic blood transfusion: systematic review and meta-analysis of randomised clinical trials. BMJ. 2013;347:f4822. Full pdf
Prick BW, Steegers EA, Jansen AJ, et al. Well being of obstetric patients on minimal blood transfusions (WOMB trial). BMC Pregnancy Childbirth. 2010;10:83. Full pdf
Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365(26):2453-62. Full pdf
Munoz M et al. Very-short-term perioperative intravenous iron administration and postoperative outcome in major orthopedic surgery: a pooled analysis of observational data from 2547 patients. Transfusion. Dec 27, 2012. Full pdf
Additional FOAMed Resources
Bloody Easy 3 – Blood Transfusions, Blood Alternatives & Transfusion Reactions: A Guide to Transfusion Medicine. 3rd edition. 2008. Ontario Regional Blood Coordinating Network. Full PDF
Dr. Helman, Dr. Himmel, Dr. Lin & Dr. Callum have no conflicts of interest to declare
Now test your knowledge with a quiz.

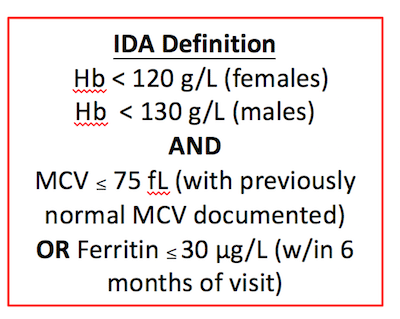
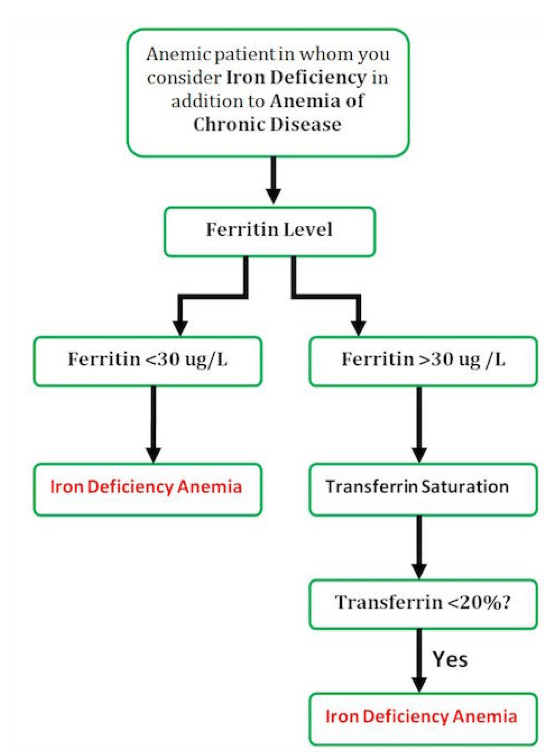



Of note, the Ministry of Health of Ontario does not recommend ordering percent iron saturation (transferrin saturation) in addition to ferritin (often cancels tests unless clear indication given) in the work up of anemia. We can take a history for anemia of chronic disease, and I simply start a trial of iron to target a ferritin of ~ 100 (and skip measuring the percent iron saturation which is sensitive to time of day, recent iron ingestion and may low in anemia of inflammation due to hepcidin causing a functional iron deficiency).
Thanks for your comments. Here is Dr. Callum’s response:
We whole heartedly agree with your concern over the overuse of iron tests. In an otherwise well patient with anemia in an outpatient clinic, a ferritin should be the only iron test ordered during the initial work-up of anemia. Some have even argued that a ferritin is overkill in young women with menorrhagia with a microcytic anemia who are low risk for thalassemia trait or have a previously normal MCV on record. We also agree that a trial of iron for patients with anemia and a ferritin below 100 is a reasonable strategy for patients with mild anemia (100-130 g/L) where there are no time pressures to fix the anemia (no planned surgery, no bleeding, no severe anemia, no need to urgently make the diagnosis).
In the severely anemic patient that presents to the ED with hemoglobin levels <90-100 g/L, there are greater time pressures to intervene to (1) make the patient feel better, (2) get the hemoglobin back up so that they get out or stay out of the transfusion zone. Here is where we recommend using the iron saturation for some patients before reaching for iv iron. We would be reluctant to recommend giving iv iron to every anemic patient with a ferritin of <100 without verifying that the severe anemia was very likely from iron deficiency. The need for the iron saturation test should be uncommon. First, most patients will have ferritin levels already measured by their family MD and be found to be <30 when they have severe anemia so no further testing will be required. Second, there will be a group of patients with severe anemia, low MCV, previously normal MCV, low risk for thalassemia trait, and a clear source for blood loss (menorrhagia) that no iron testing is required at all before iv iron. But there will be a group of anemic, complicated, usually elderly patients with severe normocytic anemia with ferritins between 30 and 100 where you won't be sure what to do without an iron saturation. If the iron sat is <20% then iv iron and then follow-up in clinic is an appropriate first step. But if the iron saturation comes back and is normal - then you need to think about other causes of anemia. In these cases we would do further investigations to determine the cause of the anemia before reaching for iv iron.
Hello. Thank you so much for another excellent episode – fantastic work everyone! I just have a couple of quick questions.
1. My hospital has a protocol for both Feraheme & Venofer infusions that say MRI results can be altered up to 3 months post Fe infusion. Can you please comment with regards to this
2. My hospital’s protocol recommends 2 doses
Feraheme has superparamagnetic properties (see product monograph http://www.takedacanada.com/ferahemepm/~/media/countries/ca/files/product%20pdfs/ferahemepm_eng_2014jun05.pdf) and may transiently affect the diagnostic ability MR imaging for up to 3 months particularly liver imaging. Most institutions that issue Feraheme typically give the patient a wallet card to carry with them with this advisory and the date of Feraheme administration. It is important to either perform MRI imaging before Feraheme administration or ensure you consult the radiologist prior to MRI imaging if Feraheme has been given in the past 3 months to determine if a different modality is preferred. Feraheme does not interfere with CT scan. In contrast, Venofer does NOT affect MRI imaging.
Sorry posted before finishing last question…
2. My hospital protocol recommends 2 doses of Feroheme spread 7-10 days apart as baseline treatment and to STOP po Fe for 5 days after infusion. Can you please also comment to this point?
Many thanks!
Irene
The dose of iron replacement depends on the severity of iron deficiency. There are formulas to calculate the amount of iron required for replacement e.g. Ganzoni formula: Weight (kg) x (target Hb g/dL – actual Hb g/dL) x 2.4 + iron stores (mg). So in a 70 kg patient with a Hb of 9 g/dL (90 g/L) with a target Hb of 12 g/dL (120 g/dL) and a desire for 500mg of iron stores, this patient would require 1004mg of iron replacement. I typically use a ball park figure of 200mg iron for every 10 g/L of Hb rise. This will replace the amount of iron for hemoglobin recovery. Then I also add ~500mg for iron stores.
So in some cases, a patient may require an initial dose of iv iron to get them out of the danger zone of requiring transfusion and then further replacement may be done using oral iron if tolerated and effective. If they’ve not tolerated oral iron, then they may need 2 or even 3 doses of iv iron depending on their iron deficit.
The product monograph for Feraheme (http://www.takedacanada.com/ferahemepm/~/media/countries/ca/files/product%20pdfs/ferahemepm_eng_2014jun05.pdf) states that the second dose can be given 2 to 8 days later. In my practice, we have typically given Feraheme a week apart.
For oral iron, I don’t think there are specific recommendations of what to do during iv iron therapy. I do typically stop the oral iron while on iv iron therapy because the amount of oral iron absorption is quite minimal (only 10-15% of oral elemental iron is absorbed) compared to iv iron. But once iv iron is completed, then I recommend re-starting oral iron the following day.
Himmel’s Top 20 Do’s and Don’ts for Anemia in the ED
1. Don’t give needless blood. Young women who are healthy can tolerate Hb of down to 50g/L if bleeding has stopped or menorrhagia is managed. Older folks (>80g/L) with multiple risk factors for heart disease do tolerate Hb of 80g/L.
2. Anemia is NOT good but Don’t give lots of blood as this does not help the underlying problem – blood is a Band-Aid solution.
3. Don’t forget that some one has to look at the etiology at some point.
4. Don’t miss thalassemia. It is wide spread.
5. Do give good advice regarding oral iron. Once per day, on an empty stomach with water (tons of patients are still told to take it with food to minimize GI side effects, believe me (I ask all the time).
6. Do give vitamin C 500mg along with oral Iron to maximize absorption in the gut.
7. Don’t forget that iron deficiency causes lots of problems other than anemia such as cognitive impairment and, in children, learning problems.
8. Do look at the RDW and the RBC count to determine whether a patient has iron deficiency anemia or thalassemia or something else.
9. Do communicate to the Family MD, or consultant, or clinic.
10. You would be amazed as to how many patients are NOT prescribed FE for Iron deficiency. Do discuss compliance and do not assume someone else will do it.
11. Do arrange follow-up. Some patients may require repeated doses of IV iron.
12. Don’t forget that iron may cause a positive stool for occult blood.
13. Do order a serum ferritin when you are not sure about the diagnosis of iron deficiency anemia. Serum ferrtin < 30 is a sensitive and specific marker for iron deficiency anemia BUT not in people with inflammation and especially NOT in people with chronic renal disease. These patients may be iron deficient and have ferritin of >100.
14. Don’t give blood just because the patient gets blood every month. Seniors from nursing homes with iron deficiency anemia need a systems approach. After the 2nd or 3rd visit to the ED for blood some one has to come up with a plan.
15. In those with inflammation (high hepcidin level), oral iron may not work and they may still benefit from IV iron. This is probably NOT a decision for the ED but someone has to arrange the referral. Don’t leave it up to someone else.
16. Pearl: in the elderly with anemia (perhaps 15 % of all elderly): 1/3 deficiency (fe, b12, folate),1/3 inflammation, and 1/3 unknown. Some one has to arrange a follow-up. Don’t ignore the anemia.
17. With iron deficiency and a HG < 90g/L (this is very significant anemia for the long term although it generally does not require transfusion), the patient needs to about 1000mg or more of absorbed ELEMENTAL iron plus daily requirement. This will take 6 months or more. Iron sulfate is only 30 % elemental iron and only 10% is absorbed if not less in ill patients. So, it takes time and persistence. Do counsel patients that it may take months of treatment. 18. Each group needs to think this out. Giving iron blindly is not good; giving blood blindly is really bad. Do meet with your ED group and hematologists and come up with an a agreed upon protocol. 19. If the patient is really anemic (<90g/L) and iron deficient, I have NO problem giving Iron sucrose in the ED (300 mg) and arranging a follow-up. This is the right thing to do. If you choose to send them to a clinic, Do warn them of the cost. Clearly, this is new knowledge for the community and will really require educational and political influence and power. 20. Remember, you may get some hostile push-back from those who sent the patients for BLOOD and from the patients who may believe that they will die without two or three units of blood. Do take time for good patient-centered care.
[…] you give blood products, you need to hear Emergency Medicine Cases discuss when IV iron is a better option in many patients. […]
Really appreciate this podcast. I’d like to point out that 100mg of Iron Sucrose can be given directly into the vein over 5 – 10 minutes. This has been done for years in dialysis sessions. Also, Iron Sulfate is notorious for causing direct and severe vomiting after taking on empty stomach.
I would like to know the reference for expected incidence of severe anaphylactic reactions. A former student sent me this link and I think it’s terrific … I have promoted use of IV iron as an internist for some time and run into a lot of fear and resistance. However, I did encounter one apparent severe anaphylacic reaction to IV iron dextran (LMW) about 6-7 years ago. The patient stood to benefit greatly from iron repletion, even at advanced age, as he had a leaking and untreatble large colon cancer … but about 15-30 minutes after the test dose, his BP crashed, and he developed severe abdominal pain … this passed after IV saline and time and I did not have to give adrenalin. If this happened to only 1:1 million people, it is unlikely I would have encountered this. I estimate I have given IV iron to about 100 people maximum.
Generally, a fine article.
Tom Perry, M.D., FRCPC
Thanks for your query. The latest and most robust data on incidence of anaphylaxis for IV iron is from the article ‘Comparative Risk of Anaphylactic Reactions Associated With Intravenous Iron Products’.
http://www.ncbi.nlm.nih.gov/pubmed/26575062 I generally avoid feraheme as it has a higher incidence of anaphylaxis compared to venofer. I think it’s also important to be note that ANY patient with a low BP with anaphylaxis should receive adrenaline. There are no contraindications to adrenaline for patients with anaphylaxis in shock. The most common cause of death in anaphylaxis is failure to give adrenaline. Hope this is helpful and thanks for listening!
Can iv iron sucrose be administered an hour or so after a rbc transfusion?
Yes – this is done regularly by our hematology colleagues. They run them back to back with a NS flush!
What if a patient has low ferritin (6), 10.0 hgb, and no iron panel was done due to low ferritin, but the patient has a genetic (parent has iron overload) and personal history of iron overload for the past 20 years?