Ashleigh Tuite infectious diseases mathematical modeler discusses factors predicting spread of SARS CoV-2, prediction models and flattening the curve of COVID-19. Is the COVID-19 virus likely to mutate? And if it does what are the most likely consequences? What do prediction models show in terms of how long this pandemic will last? Is there likely to be a 2nd peak?…
Podcast production, sound design & editing by Anton Helman
Written Summary and blog post by Anton Helman March, 2020
Cite this podcast as: Helman, A. Tuite, A. Episode 141 COVID-19 Part 5 – Epidemiology and Prediction Models. Emergency Medicine Cases. March, 2020. https://emergencymedicinecases.com/covid-19-epidemiology-prediction-models. Accessed [date]
This podcast and blog post are based on Level C evidence – consensus and expert opinion. Examples of protocols, checklists and algorithms are for educational purposes and require modification for your particular needs as well as approval by your hospital before use in clinical practice.
Key COVID-19 epidemiologic measurements
Based on a systematic review published March 31st, current evidence suggests the following key COVID-19 epidemiologic measurements:
- Doubling time: 3-7 days for the epidemic to double in size
- Reproduction number – R0 (number of expected secondary cases arising from a single individual, a measure of the transmissibility of a disease): ranges from 1.9 to 6.5, probably between 2.0 and 3.0 (comparable to SARS-CoV, HINI outbreak and a bit more than seasonal influenza which is thought to be about 1.5)
- Incubation period: 4-6 days (similar to SARS Co-V and MERS)
- Serial interval: 4-8 days
- Case fatality rate: ranged from 0.3% to 1.4% (outside China)
- Duration of viral shedding: highly variable ranging from 8 to 37 days (viral RNA levels may be higher early after symptom onset)
Johns Hopkins University interactive up-to-date map that tracks global transmission.
Is SARS Co-V2 likely to mutate and become more virulent? Is there immunity post-infection?
When SARS Co-V RNA virus replicates it is very error prone, so it does mutate, with estimated mutation rate of 1-2 per month (slower than influenza) however most mutations will be neutral and will not effect how the virus behaves. The virus will likely be relatively stable over the next few months and is unlikely to mutate into a more virulent and deadly virus, and we will likely be able to develop an effective vaccine within 15-18 months. Weak evidence suggests that some of the antibodies that are induced in those with COVID-19 are protective but it is not known whether all infected patients mount a protective immune response and how long a protective effect might last.
According to the CDC it is unclear if there is immunity post-infection.
- Immunity post-infection is likely to occur but is unproven
- It is unclear how long immunity lasts as antigenic drift may occur
- Many expert virologists believe that recovered patients (including health care workers) will have at least short-term immunity
Which preventative measures help the most to curb transmission of SARS-CoV2?
One of the drivers of the Reproduction number – R0 is the number of contacts people in the region each have per day. A combined strategy of workplace distancing, quarantine of exposed individuals (and less so) school closures and airport screening, seem to be highly effective at containing spread, however the effectiveness of these control measures could be curtailed by a significant portion of asymptomatic patients (17.9%), and pre-symptomatic transmission (12.1%).
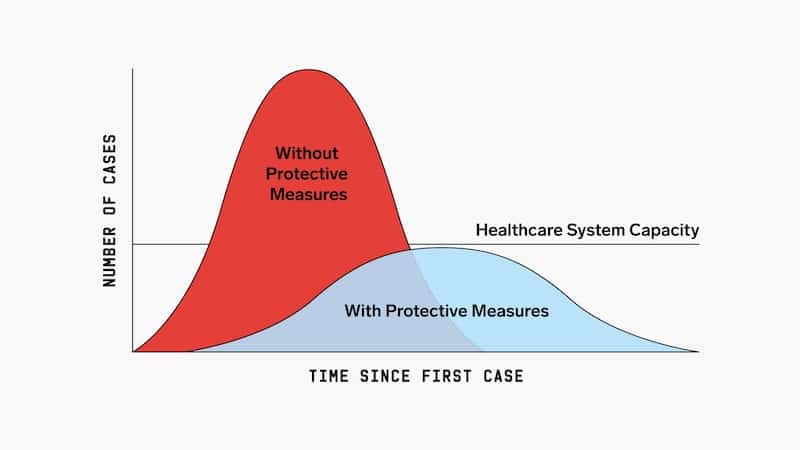
Responsive social distancing plan
According to University of Toronto mathematical prediction models, the worst case scenario (limited measures to curb transmission and minimal testing), 50-70% of the population of Ontario would contract the virus and the number of ICU beds required would be 20 times the current maximum capacity. Strict social distancing would need to be in place for 6-12 months to stop ongoing transmission. However, if we adapt a flexible plan whereby we employ social distancing for a few weeks initially, followed by responsive social distancing or staggered return to work/school (with the trigger for social distancing being ICU bed capacity), the mathematical model would predict that the rate of illness would be low enough that we would safely be able to return to work/school (at least partially).
A study of the COVID-outbreak in China showed that social distancing measures were most effective if the staggered return to work occured at 3-4 months after the outbreak started. Apparently staggered return to working at this interval reduced the median number of infections by more than 92%.
Community based measures to mitigate the spread of COVID-19 in Canada – Government of Canada
The initial stricter social distancing and isolation of symptomatic patients will “flatten the curve” and buy us time to increase resources and increase health care capacity to meet needs:
- Buy more ventilators
- Open respiratory isolation wards
- Stockpile PPE
- Integrate telemedicine
- Expand lab testing capacity
- Train emergency providers
- Learn more from colleagues in hotspots
CAIC-RT: COVID-19 Acute and Intensive Care Resource Tool estimates the maximum daily number of incident COVID-19 cases that a healthcare system could manage based on age-based case distribution and severity, and the number of available acute and critical care resources.
Is there likely to be a 2nd peak? Is COVID-19 likely to be a seasonal illness?
Whether on not summer will attenuate the spread of CoV-2 in the northern hemisphere is unknown. If it is attenuated in warmer weather there may be a 2nd peak in the fall similar to SARS CoV-1. A second peak may also occur if social distancing measures that have been lifted are not implemented soon enough after the case rate starts to increase.
References
- Lipsitch M, Swerdlow DL, Finelli L. Defining the Epidemiology of Covid-19 – Studies Needed. N Engl J Med. 2020;382(13):1194-1196.
- Prem K, Liu Y, Russell TW, et al. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Health. 2020;
- Park, M.; Cook, A.R.; Lim, J.T.; Sun, Y.; Dickens, B.L. A Systematic Review of COVID-19 Epidemiology Based on Current Evidence. J. Clin. Med. 2020, 9, 967.
- Mizumoto, K.; Kagaya, K.; Zarebski, A.; Chowell, G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Eurosurveillance 2020, 25.
- Du, Z.; Wang, L.; Xu, X.; Wu, Y.; Cowling, B.J.; Meyers, L.A. The serial interval of COVID-19 from publicly reported confirmed cases. medRxiv 2020.
- Nishiura, H.; Linton, N.M.; Akhmetzhanov, A.R. Serial interval of novel coronavirus (2019-nCoV) infections. medRxiv 2020.
- National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases. Coronavirus Disease 2019 (COVID-19). Healthcare Professionals: Frequently Asked Questions and Answers. CDC 24-7 Organization website. https://www.cdc.gov/coronavirus/2019-ncov/hcp/faq.html Published/Update Febraury 21, 2020. Updated March 22, 2020. Accessed March 30, 2020.
- Greenfieldboyce, N. Do You Get Immunity After Recovering From A Case Of Coronavirus? March 20, 2020. https://www.npr.org/sections/goatsandsoda/2020/03/20/819038431/do-you-get-immunity-after-recovering-from-a-case-of-coronavirus. Accessed March 28, 2020.
- Del Rio C, Malani PN. COVID-19-New Insights on a Rapidly Changing Epidemic [published online ahead of print, 2020 Feb 28]. JAMA. 2020;10.1001/jama.2020.3072. doi:10.1001/jama.2020.3072.
- Li Q, Guan X, Wu P, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia [published online ahead of print, 2020 Jan 29]. N Engl J Med. 2020;10.1056/NEJMoa2001316. doi:10.1056/NEJMoa2001316.
- Report of the WHO-China Joint Mission on Coronavirus DIsease 2019 (COVID-2019). February 16-24, 2020. http://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf (Accessed on March 04, 2020).
- Liu Y, Yan LM, Wan L, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis 2020.
- Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N Engl J Med 2020; 382:970.
- Yu P, Zhu J, Zhang Z, et al. A familial cluster of infection associated with the 2019 novel coronavirus indicating potential person-to-person transmission during the incubation period. J Infect Dis 2020.
- Bai Y, Yao L, Wei T, et al. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA 2020.
Drs. Helman and Tuite have no conflicts of interest to declare




Thank you Anton for the comments you made before the start of the interview of Part 5. I am getting ready for my overnight shift in the ED in Silver Spring, Maryland (Suburb of Washington DC). I appreciate your candor, as I find myself randomly tearing up from stress, insomnia, fear for myself, family and patients.