Our patient from part 1 of this 2-part podcast series on pulmonary embolism management is now tanking. Recall she is a 30-year-old female on oral contraceptive pill who was satting 68% on room air when EMS picked her up after 6 hours of shortness of breath and a syncopal episode. She had an initial ECG and PoCUS suggestive of right heart strain, was started on IV heparin and had a saddle embolus on CTPA.
This time, however, instead of just being admitted to the ICU, she comes back from the donut of truth satting 88% on a non-rebreather with a blood pressure of 70/40 and now she’s altered. She now has a high-risk pulmonary embolism. How is this going to change our management?
These are the sickest of the PE patients – about 5-10% of all PE cases, but with a high mortality rate of 30-40% at 30 days. All of these patients, unless there are absolute contraindications, should be considered for timely thrombolysis, which is guideline recommended despite the evidence for mortality benefit being fair at best. Just like in intermediate-risk patients, high-risk PE patients are a heterogenous group ranging from the patient with persistent systolic BP under 90 and otherwise looking not bad, to the cardiac arrest patient. There are many nuances in the management of these patients in oxygenation and airway management, hemodynamic support, acid/base management, thrombolysis, catheter-directed therapies that we dive into with our guest experts Dr. Lauren Westafer, Dr. Bourke Tillmann and Dr. Justin Morgenstern…
Podcast production, sound design & editing by Anton Helman; Voice editing by Braedon Paul
Written Summary and blog post by Sara Brade, edited by Anton Helman May, 2025
Cite this podcast as: Helman, A. Morgenstern, J. Tillmann B. Westafer, L. High Risk Pulmonary Embolism Management. Emergency Medicine Cases. May, 2025 https://emergencymedicinecases.com/high-risk-pulmonary-embolism-management. Accessed June 2, 2025
Part 1 of this 2-part podcast is on Intermediate Risk PE Risk Stratification and Management
How does PE kill our patients? The Pulmonary Embolism Spiral of Death
In pulmonary embolism, there is a physical obstruction (ie. clot) decreasing the flow from RV to LV. The RV is a weak muscle to begin with and this obstruction increases the pressure the RV has to pump against. As the RV starts to fail, it dilates and becomes even weaker resulting in hypotension. In an undifferentiated patient, we’re likely to start by giving them fluids. But the RV is very sensitive to fluids and as we give more fluids to a patient in this state, this causes the RV to dilate further. The RV then becomes ischemic and bows into the LV. The LV now can’t fill well because the RV is in the way and because there is limited flow coming from the pulmonary vessels. This worsens systemic hypotension. Further, vasoconstriction in areas of the lungs obstructed by clot causes hypoxemia, and hypoxemia worsens myocardial perfusion. This cycle is how our high risk pulmonary embolism patients die from if we don’t intervene.
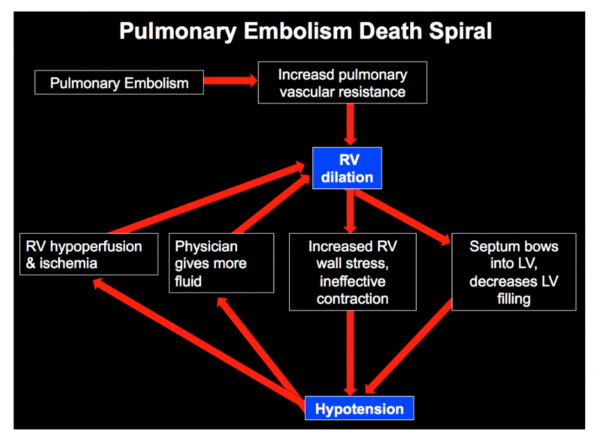
Image soure: EMCrit : https://emcrit.org/pulmcrit/eight-pearls-for-the-crashing-patient-with-massive-pe/
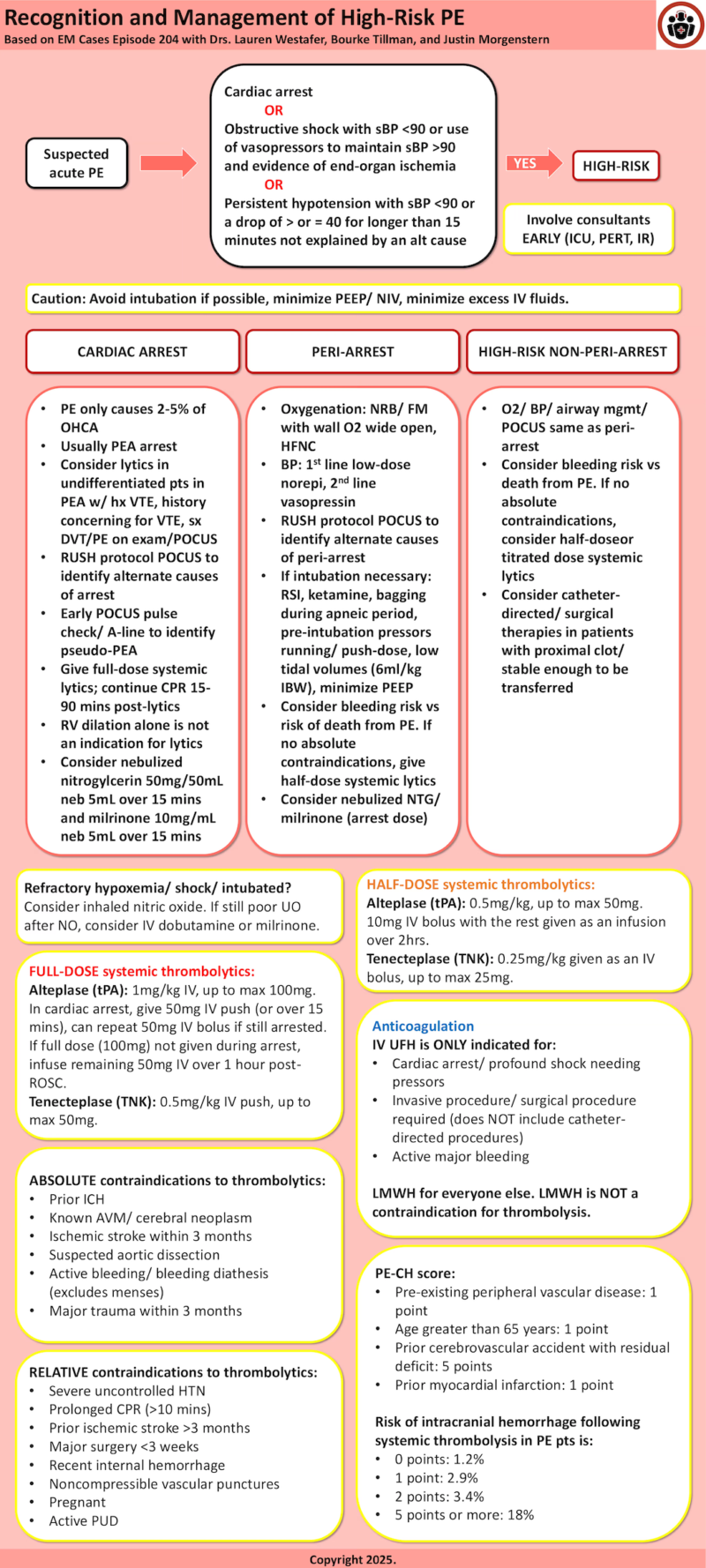
Defining high-risk Pulmonary Embolism
High-risk pulmonary embolism, previously called massive PE, includes patients with hemodynamic instability due to PE delineated by one of the following:
- Cardiac arrest
- Presence of obstructive shock with a systolic BP <90mmHg or use of vasopressors to maintain a BP >90mmHg AND evidence of end-organ ischemia (ie. altered mental status, cool skin, oliguria, increased lactate)
- Persistent hypotension with a systolic BP <90mmHg or a drop of 40 mmHg or more for longer than 15 minutes not explained by an alternative cause (ie. sepsis, hemorrhage, etc).
Heterogeneity within the high-risk group informs management decisions
Like the intermediate-risk pulmonary embolism group, the high-risk group encompasses a wide spectrum of patients from cardiac arrest to a patient who looks okay clinically but has a mildly elevated lactate and a soft BP. Group these patients broadly into three groups to help guide management:
- Cardiac arrest
- Peri-arrest
- High-risk non-peri-arrest – note that oxygenation status alone is not captured in the classic high-risk definition schema but consider patients with high oxygen needs in this group.
Cardiac Arrest: Pulmonary Embolism Special Considerations
PE is estimated to cause 2-5% of all out-of-hospital cardiac arrests.
Presence of a shockable rhythm and no prior history of VTE has a NPV of 98% for excluding PE as the cause of cardiac arrest. PE most often causes PEA arrest.
When patients come to the emergency department in cardiac arrest, we often do not have a preceding diagnosis of PE. It may be reasonable to consider empiric thrombolytics in patients in PEA with multiple VTE risk factors, history of PE, history suspicious for PE (ie. sudden onset dyspnea pre-arrest), and/or signs on exam/ PoCUS of DVT. PoCUS can also be used to assess the heart/ IVC for signs of PE/ obstructive shock, but RV dilation alone is not an indication for thrombolytics. PoCUS pulse checks/ arterial line placement can also help differentiate true PEA from pseudo-PEA, and the latter can be managed as profound hypotension.
While some evidence suggests quite favourable outcomes for patients given thrombolytics in arrest due to PE (up to 87% alive at 3 months), these numbers may be inflated due to selection bias. When selecting cardiac arrest patients for thrombolytics, it is important to consider baseline status and duration of arrest. Thrombolytics will likely have a more favourable outcome in patients with a short duration of arrest and minimal downtime.
There is no high-quality evidence to guide us on how long to continue CPR for post-lytics. Some experts recommend at least 15 minutes, while European guidelines recommend 60-90 minutes. We know that, in general, outcomes are very poor for cardiac arrests of prolonged duration. These guidelines can make the decision about when to terminate resuscitation challenging.
Peri-arrest Pulmonary Embolism Initial Management
These sick peri-arrest patients often come to us undifferentiated, and so manage the initial stages like any other resuscitation. However, in undifferentiated shock patients, we should force ourselves to consider, “Could this be an RV issue?” Consider using PoCUS to perform a RUSH protocol to help narrow your differential diagnosis and to evaluate for signs of hemodynamically significant PE/ RV compromise. In patients with known PE, evaluate whether it’s the PE causing shock or if there is an alternate cause (ie. sepsis, hemorrhage). Avoid anchoring on the PE diagnosis as the sole cause of instability.
In peri-arrest PE patients, there are 3 key initial management considerations that differ from other resuscitations:
- Avoid intubation if possible – In general, try to avoid intubation/ NIV in these patients. The transition to positive pressure can worsen RV performance and lead to cardiac arrest. Flush rate non-rebreather/ high-flow nasal cannula (HFNC) are the preferred oxygenation strategies for these patients.
- Minimize PEEP – If these patients do end up requiring intubation/ NIV, excessive PEEP can further worsen RV hemodynamics. Optimal PEEP value for these patients is not known. Target the level of PEEP required to optimize gas exchange and minimize hypoxic vasoconstriction.
- Minimize IV fluids – Excessive IV fluid distends the RV and leads to worsening RV cardiac output, myocardial ischemia, and LV filling. Use IV fluids judiciously in these patients.
Peri-arrest Pulmonary Embolism Oxygenation and Airway Management
It’s critical to avoid hypoxemia in pulmonary embolism patients as it can precipitate the RV death spiral. For patients who require more than nasal prong oxygen, wall O2 wide open + nonrebreather (wall rate up to 40L/min ie. flush rate) may be more effective than HFNC as many patients are mouth breathers. HFNC is favoured over NIV.
Despite maximal O2 support, your patient may still require intubation or NIV. While awake intubation or ketamine-assisted may be a good option in theory, this can be practically challenging to execute in the peri-arrest patient. Consequently, our experts recommend intubation using RSI technique with drugs that are familiar to you/your team. Bagging during apenic period is critical to avoid hypoxemia/ peri-intubation arrest. Starting pre-intubation vasopressors and having push-dose at the bedside are also recommended to avoid worsening hypotension/ arrest. Consider LMA as a rescue device in appropriate patients.
Once intubated, optimize ventilator settings to minimize hemodynamic effects on the RV: target low tidal volumes (6ml/kg ideal body weight) and minimize PEEP.
Peri-arrest Pulmonary Embolism: BP Management/Choosing a Vasopressor
BP management in these patients should be guided by the primary driver of hypotension. We have to ask ourselves again whether the pulmonary embolism is the sole cause of instability. PoCUS findings and CT clot burden (if available) may be helpful in this regard.
For patients requiring vasoactive BP support, our experts recommend picking the pressor your team is most familiar with to increase speed/ safety of pressor delivery. In most cases, this will be low dose norepinephrine. While there is some theoretical concern with norepinephrine increasing pulmonary vascular resistance in high doses and some animal studies suggesting benefits with first-line vasopressin, there are no high-quality RCTs in humans to date to guide our management. If shock refractory to low dose norepinephrine, do not crank up the dose – instead, consider adding vasopressin. Other experts have recommended vasopressin as a first line agent due to concerns that norepinephrine in high doses causes increased pulmonary vascular resistance.
Thrombolytic choice/ dosing in high risk pulmonary embolism management
Generally, full dose thrombolytics should be reserved for patients in whom pulmonary embolism is deemed to be the primary cause of cardiac arrest. Our experts recommend half-dose thrombolytics or titrated dose thrombolytics for all other high risk pulmonary embolism patients.
Cardiac arrest pulmonary embolism thrombolytic dosing
Full-dose systemic thrombolytics
- Full-dose alteplase (tPA): 1mg/kg IV, up to max 100mg. In cardiac arrest, give 50mg IV push (or over 15 mins), can repeat 50mg IV bolus if still arrested. If full dose (100mg) not given during arrest, infuse remaining 50mg IV over 1 hour post-ROSC.
- Full-dose tenecteplase (TNK): 0.5mg/kg IV push, up to max 50mg.
- <60kg: 30mg
- 60-70kg: 35mg
- 71-80kg: 40mg
- 81-90kg: 45mg
- > or = 90kg: 50mg
- There is no high-quality evidence to suggest how long to continue CPR for post-thrombolytics. European guidelines recommend 60-90 minutes. Some experts recommend at least 15 minutes. Consult your local guidelines/ experts.
- TNK may portend a higher risk of bleeding compared to tPA. The choice between alteplase and tenecteplase will likely be based on institutional availability, although most of the current evidence in PE is for alteplase.
Peri-arrest pulmonary embolism thrombolytic dosing: half-dose thrombolytics
Consider half-dose systemic thrombolytics.
- Half-dose alteplase: 0.5mg/kg, up to max 50mg. 10-20mg bolus with the rest given as an infusion over 2hrs.
- Half-dose tenecteplase: 25mg/kg given as a single bolus, up to max 25mg.
High-risk non-peri-arrest pulmonary embolism thrombolytic dosing
- Consider half-dose systemic thrombolytics as outlined above.
- Consider titrated dosing of systemic thrombolytics in patients who are less sick/ you feel there is more time to manage.
Pitfall: Only 1/3 of eligible patients with high-risk PE receive systemic thrombolytics in observational studies.
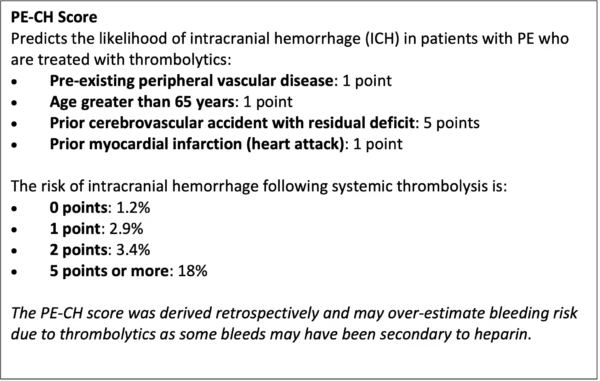
Risk-benefit Analysis for Thrombolysis and Catheter-based Treatment Decisions in Pulmonary Embolism: PE-CH Score and Contraindications to Thrombolytics
Our experts use a gestalt-based risk-benefit approach and shared decision-making conversations to guide thrombolysis decisions in patients with pulmonary embolism. In discussion with your patient and/or their family, consider how the risk of intracranial hemorrhage (ICH) and potential functional deficits compares to the risk that the patient may die from their PE.
The patient’s age, the PE-CH (see below), and reviewing the patient’s absolute/ relative contraindications to thrombolysis can help to inform your clinical impression of a given patient’s risk of ICH. Also consider your practice location and your access to catheter-directed/ surgical therapies. Discuss with your local PERT/ critical care team to review management options.
Remember also that not all absolute contraindications are created equal. For example, someone with brain metastases is very likely to develop ICH post-lytics, but someone with a remote history of an intracranial bleed may have a low chance of bleeding. Further, we are used to talking about risk of ICH with thrombolytics in the context of stroke where the risk of ICH is much higher than in PE.

Source: RebelEM https://rebelem.com/rebel-review/rebel-review-37-contraindications-to-thrombolytics/contraindications-to-thrombolytics/
Anticoagulation Choices in High Risk Pulmonary Embolism Management: LMWH is first line for most patients
Low-molecular weight heparin (LMWH) is preferred in most patients because of its more favourable bleeding profile/ predictability compared to unfractionated heparin (UFH).
IV UFH is recommended in the following situations:
- Cardiac arrest or peri-arrest
- Persistent shock (as subcutaneous route is unlikely to be well-absorbed)
- Invasive procedure (eg LP) or surgery (eg appendectomy) is required (this does not include mechanical embolectomy/catheter-based PE procedures)
- Active major bleeding
LMWH use is not a contraindication to thrombolysis or catheter directed therapies!
In patients receiving thrombolysis, anticoagulation should be resumed or started after a minimum of 2 hours. Some recommend waiting up to 6 hours to minimize the risk of bleeding complications. This timing decision can usually be left to the interventional/ ICU team.
Catheter-directed Therapies in the Management of Pulmonary Embolism: Indications?
High-risk pulmonary embolism is a challenging disease to study in a randomized fashion due to the population’s heterogeneity/ acuity. As such, there are no large high-quality RCTs comparing systemic thrombolysis to catheter/ surgical-based therapies for PE. The data we do have is largely observational and heavily funded by industry.
Catheter-directed therapies can be considered in patients who:
- Are well enough for transfer
- Do not require immediate thrombolysis
- Are at high risk for major bleeding complications
- Have a proximal clot on CT pulmonary angiogram
Catheter-directed therapies include mechanical thrombectomy or catheter-directed thrombolysis. In rare circumstances like failed thrombolysis or clot-in-transit, surgical embolectomy may be offered. In the experience of our experts, most patients receive mechanical thrombectomy over catheter-directed thrombolysis.
Acid-Base Management in High Risk Pulmonary Embolism Management
To keep the pulmonary arteries open, avoid hypoxia, hypercapnia, and avoid acidosis. PE leads to VQ mismatch causing hypoxic vasoconstriction and this impairs gas exchange. These patients are not acidotic due to lack of bicarbonate and so bicarbonate boluses/ infusions will not likely lead to clinical improvement. The volume of IV fluid associated with giving bicarb may also negatively impact RV function. Unfortunately, there is no high-quality evidence to guide this decision specifically in PE patients; however, there is some evidence to suggest giving bicarb improves outcomes in patients with low bicarb, acidosis, and renal failure. Most of our PE patients presenting acutely unwell to the ED with a high-risk PE will not have had the time to develop renal failure, and so there is likely no role for giving bicarb to these patients in the ED.
Pulmonary Vasodilation in the Management of High Risk Pulmonary Embolism: Nitric Oxide, Milrinone, Dobutamine, Nitroglycerin
There may be a role for medications to dilate the pulmonary arteries in high-risk PE based on limited data. Our ICU expert recommends inhaled nitric oxide (NO) for PE patients who have refractory hypoxemia/ shock/ are intubated. If despite inhaled NO these patients still have poor urine output, dobutamine or milrinone can be considered.
Other experts have recommended nebulised nitroglycerin (50mg/50mL nebulise 5mL over 15 mins) and/or nebulised milrinone (10mg/mL nebulise 5mL over 15 mins) in cardiac arrest and peri-arrest PE patients based on limited data.
Pulmonary embolism response teams (PERT): Good or Bad?
PERT implementation has been shown to decrease 30-day mortality for the sickest patients and decrease the time to patients receiving anticoagulation. PERTs are associated with an increased use of catheter-directed therapies which have little evidence to support them over systemic thrombolysis. PERTs are costly and their widespread implementation could mean EM physicians become less skilled in managing sick PE patients.
Key take home points for the management of high-risk PE
- Think about high-risk PE in 3 categories: cardiac arrest, peri-arrest, high-risk non-peri-arrest
- Three key considerations in the initial management stages for high-risk PE: avoid intubation if possible, minimize PEEP/ NIV, minimize IV fluids
- To oxygenate these patients, start with facemask non-rebreather with wall O2 cranked to the max. If this fails, move to HFNC
- In high-risk PE, use a cognitive forcing strategy to consider whether the PE is the sole cause of instability or if there other factors at play. Use PoCUS to rule screen for other causes.
- For BP support, reach for low dose norepi 1st line and add vasopressin 2nd line if needed.
- Consider inhaled nitric oxide, milrinone, nitroglycerin to dilate pulmonary arteries in peri-arrest patients
- Thrombolytics are indicated in high-risk PE. Give full-dose thrombolytics in cardiac arrest, and half-dose otherwise. Systemic thrombolytics are favoured if the patient is peri-arrest with no absolute contraindications.
- Catheter-directed therapies may also be an option in patients with a proximal clot who are stable enough to be transferred out of the department/ your hospital and/or have contraindications to systemic thrombolysis.
- LMWH is not a contraindication to thrombolysis and is the first line anticoagulant for the majority of intermediate and high risk PE patients
- IV UFH is preferred over LMWH only for patients in cardiac arrest and peri-arrest, those going for invasive surgical procedures (not catheter-directed procedures), and those with active major bleeding.
- Anticoagulation after thrombolysis should be delayed 2-6 hours
- PE causes 2-5% of OHCA. Usually causes PEA arrests.
- Continue CPR for 15-90 mins post-lytics.
Part 1 of this 2-part podcast is on Intermediate Risk PE Risk Stratification and Management
PoCUS and PE deep dive on Quick Hits 65
References
- Bhat, T. (2015). Inhaled nitric oxide in acute pulmonary embolism – a systematic review. Rev Cardiovasc Med, 1-8.
- Brunet, S. (2017). The Pulmonary Embolism Severity Index (PESI) score and disposition decisions in Calgary emergency departments. CJEM, MP32.
- Chopard, R. (2022). Trends in management and outcomes of pulmonary embolism with a multidisciplinary response team. Journal of Thrombosis and Thrombolysis, 54: 449-460.
- Dam Lyhe, M. (2020). Pulmonary vasodilation in acute pulmonary embolism – a systematic review. Pulmonary Circulation, 1-16.
- European Society of Cardiology. (2020). 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism. European Heart Journal, 543-603.
- Farkas, J. (2023). Submassive and Massive PE. Retrieved from Internet Book of Critical Care (IBCC): https://emcrit.org/ibcc/pe/
- Gonzalez, S. (2024). Impact of a pulmonary embolism response team (PERT) in the prognosis of patients with acute symptomatic pulmonary embolism. Revista Clinica Espanola, 141-149.
- Gotzinger, F. (2023). Interventional therapies for pulmonary embolism. Nat Rev Cardio, 670-684.
- Gotzinger, F. (2023). Interventional Therapies for pulmonary embolism. Nature Reviews Cardiology, 670-684.
- Hobohm, L. (2023). Pulmonary Embolism Response Team (PERT) Implementation and its clinical value across countries: scoping review and meta-analysis. Clinical Research in Cardiology, 1351-1361.
- Hobohm, L. (2024). The Current Evidence of Pulmonary Embolism Response Teams and Their Role in Future. Hamostaeologie, 44:172-181.
- Hussein, E. (2023). Pulmonary embolism response team for hospitalized patients with submassive and massive pulmonary embolism: a single-centre experience. Journal of Vascular Surgery, 741-747.
- Jaff, M. (2011). AHA Guidelines: Management of Massive and Submassive pulmonary embolism, iliofemoral deep vein thrombosis and chronic thromboembolic pulmonary hypertension. Circulation, 1788-1830.
- Marti, C. (2015). Systemic thrombolysis therapy for acute pulmonary embolism: systematic review and meta-analysis. European Heart Journal, 605-614.
- Messika, J. (2017). Severe pulmonary embolism managed with high-flow nasal cannula oxygen therapy. Eur J Emerg Med, 24(3):230–2.
- Pandya, V. (2024). Evolution of Pulmonary Embolism Response Teams in the United States: A Review of the Literature. Journal of Clinical Medicine, 13:3984-4002.
- Perez-Nieto, O. (2023). Hemodynamic and respiratory support in pulmonary embolism: a narrative review. Frontiers in Medicine, 1-12.
- PERT Consortium. (2019). Diagnosis Treatment and Follow Up of Acute Pulmonary Embolism – Consensus Practice from the PERT Consortium. Clinical and Applied Thrombosis/Hemostasis , 25:1-16.
- Rivera-Lebron, B., & Weinberg, A. (2024). Approach to thrombolytic (fibrinolytic) therapy in acute pulmonary embolism: Patient selection and administration. Retrieved from UpToDate: https://www.uptodate.com/contents/approach-to-thrombolytic-fibrinolytic-therapy-in-acute-pulmonary-embolism-patient-selection-and-administration?search=pulmonary+embolism&source=search_result&selectedTitle=7%7E150&usage_type=default&display_rank=6
- Rojas Murguia, A. (2024). Reduced-Dose Thrombolysis in Acute Pulmonary Embolism: A systematic review. Angiology, 208-218.
- Rouleau, S., Casey, S., Kabrheal, C., Vinson, D., & Long, B. (2024). Management of high-risk pulmonary embolism in the emergency department: a narrative review. American Journal of Emergency Medicine, 1-11.
- Silver, M. (2023). Outcomes in high-risk pulmonary embolism patients undergoin FlowTriever Mechanical Thrombectomy or other contemporay therapies: FLAME Study. Circulation, 16:10.
- Thrombosis Canada. (2023). Pulmonary Embolism Treatment. Retrieved from Thrombosis Canada: https://thrombosiscanada.ca/clinical_guides/pdfs/44_53.pdf
- Thrombosis Canada. (2023). Venous Thromboembolism: Duration of Treatment. Retrieved from Thrombosis Canada: https://thrombosiscanada.ca/clinical_guides/pdfs/DURATIONOFANTICOAGULANTTHERAPY_80.pdf
- Vedovati,C. (2012). Multidetector CT scan for Acute Pulmonary Embolism: Embolic Burden and Clinical Outcome. Chest, 142:1417-1424.
- Watson, N. (2024). Trends in Discharge Rates for Acute Pulmonary Embolism in U.S. Emergency Departments. Annals of Internal Medicine, 177:2.
- Weinberg, A., & Rali, P. (2024). Treatment, prognosis, and follow-up of acute pulmonary embolism in adults. Retrieved from UpToDate: https://www.uptodate.com/contents/treatment-prognosis-and-follow-up-of-acute-pulmonary-embolism-in-adults?search=pulmonary%20embolism&source=search_result&selectedTitle=1%7E150&usage_type=default&display_rank=1
- Westafer, L. (2023). Managing Pulmonary Embolism . Annals of Emergency Medicine, 394-402.
- Wudwud, A. (2023). RV Failure and Acute Pulmonary Embolism. Retrieved from EMOttawa Blog: https://emottawablog.com/2023/03/right-ventricular-failure-and-acute-pulmonary-embolism/
- Zuin, M. (2024). International Clinical Practice Guideline Recommendations for Acute Pulmonary Embolism. Journal of the American College of Cardiology, 84:16.
Drs. Helman, Westafer, Tillmann and Morgenstern have no conflicts of interest to declare



Hi, in the periarrest suspected PE in witch you have a high suspicion on history, pocus, clinical finding. Would you thrombolyse without a prior scan (le’ts say your not able to stabilise the patient enough to go there safely)? Even though I think the answer is a clear yes, I can’t find it in the show note and I can’t remember it was said directly. Thank you!
Yes! Absolutely.