In this Part 1 of our two-part series on acute heart failure, Anton is joined by Dr. Tarlan Hedayati and Dr. Bourke Tillman to answer such questions as: how does PoCUS compare with clinical assessment and CXR in diagnostic accuracy for acute heart failure? How do we best integrate PoCUS in the our assessment and management of the patient with acute heart failure? What is PPV HAVoC and how can we use it to optimize acute heart failure management goals? What should be our specific goals of management in the acute heart failure depending on the underlying cause? How does high flow nasal cannula (HFNC) compare to non-invasive positive pressure ventilation (NIPPV) in the management of acute heart failure? How should we interpret the C3PO trial in the context of the world’s literature on NIPPV in acute heart failure? How should we dose nitroglycerin to maximize its effects without dumping the blood pressure in patients with SCAPE and those without SCAPE? How should we best time and dose furosemide in the acute heart failure patient with renal insufficiency? Is there any role for morphine or ACEi in the ED management of acute heart failure? What are best anxiolytic medication choices in acute heart failure? Is there any role for second line diuretics in the management of acute heart failure in the ED? and many more…
Podcast production, sound design & editing by Anton Helman
Written Summary and blog post by Kate Dillon, edited by Anton Helman December, 2021
Cite this podcast as: Helman, A. Hedayati, T. Tillmann, B. Episode 163 Acute Heart Failure ED Management – PoCUS, Oxygenation Strategies, Medication Strategies, PPV HAVoC and SCAPE. Emergency Medicine Cases. December, 2021. https://emergencymedicinecases.com/acute-heart-failure-management-pocus-oxygenation-ppv-havoc-scape. Accessed [date]
Go to part 2 of this 2-part podcast on acute heart failure
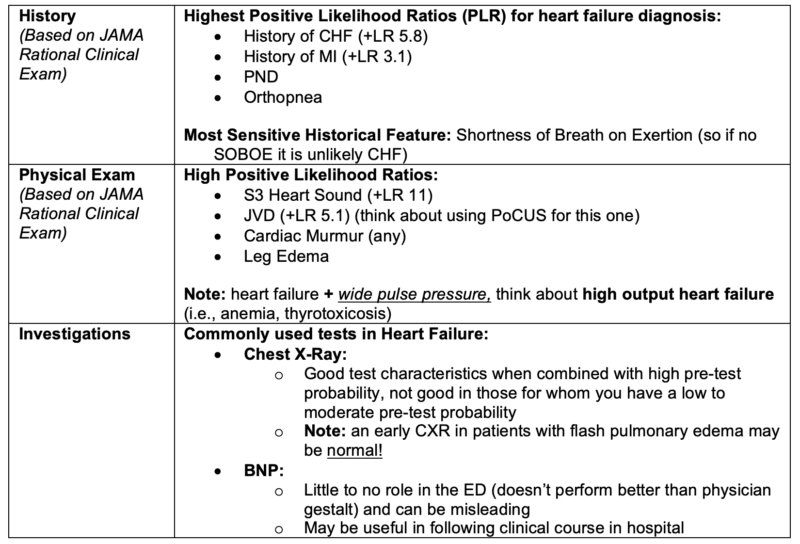
Value of clinical exam findings and basic investigations in the diagnosis of acute heart failure
Deep dive into value of BNP in ED management of heart failure: Journal Jam 12 BNP for Diagnosis of Acute CHF
PoCUS is more accurate than CXR and clinical exam in the diagnosis of acute heart failure
Assessment of the patient suspected of acute heart failure with PoCUS helps to differentiate it from other causes of shortness of breath, is accurate for the diagnosis of acute heart failure and can help elucidate the underlying cause. B-lines are only the start. Follow the links for more info on each of the PoCUS aspects of assessment of the patients with acute heart failure.
- B-Lines on PoCUS are 94% sensitive, 92% specific for the diagnosis of acute heart failure

Image: Avila, J., Kim, D. (2015, December 18). US against the World: Ultrasound in differentiating COPD from CHF. CanadiEM. Retrieved October 4, 2021, from https://canadiem.org/us-world-ultrasound-differentiating-copd-chf/.
POCUS Cases on LV Dysfunction with Rob Simard
POCUS Cases on IVC and volume assessment with Rob Simard
It is important to identify the underlying cause of acute heart failure to help guide management
While medication non-compliance and dietary indiscretion are the most common causes of acute heart failure, there are several life-threatening causes that should be identified and addressed in the ED, the most time-sensitive being ACS:
- Cardiac ischemia + acute heart failure is an indication for emergency transfer to cath lab
- Mechanical causes
- Severe aortic stenosis
- Ruptured cardiac valve
- Pericardial effusion (including tamponade)
- Myocarditis / Endocarditis
- COPD/Asthma
- Pneumonia
- High output states (wide pulse pressure is a clue)
- Severe anemia
- Thyrotoxicosis
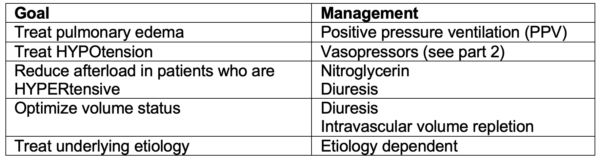
PPV HAVoC mnemonic for goals of management in acute heart failure
PPV – positive pressure ventilation
Hypotension correction with vasopressors
Afterload reduction
Volume status (consider diuresis vs. intravascular volume repletion)
Cause (treat underlying cause)
General approach to the management of acute heart failure
Divide patients into two categories based on their hemodynamic status:
- Hypotensive (Cardiogenic Shock): severe impairment of myocardial contractility most often caused by ischemia that results in diminished cardiac output, end‐organ hypoperfusion, and hypoxia, presenting with hypotension refractory to volume resuscitation requiring vasopressors and ionotropes and/or mechanical intervention. See Part 2.
- Not hypotensive (No Cardiogenic Shock): These patients will usually present HYPERtensive (can present normotensive). In this category, the primary issue is that the heart cannot overcome the increased afterload. Treatments focus on decreasing afterload. In severe cases patients can present in flash pulmonary edema or SCAPE (Sympathetic Crashing Acute Pulmonary Edema), a subset of patients with very high systemic vasoconstriction, hypertension and elevated afterload who may be hypovolemic, euvolemic or hypervolemic.
Identify and treat the underlying cause of acute heart failure in tandem
Management of acute heart failure without cardiogenic shock: Oxygenation, nitroglycerin, diuresis and treating the underlying cause
Oxygenation in acute heart failure management
Escalation of oxygenation strategies is usually indicated in the dyspneic acute heart failure patient who continues to display an oxygen saturation <91%. Options include non-rebreather, high flow nasal cannula, non-invasive positive pressure ventilation (NIPPV) and endotracheal intubation.
Non-invasive positive pressure ventilation (NIPPV): B-PAP and C-PAP
Patients with pulmonary edema from acute heart failure who are tachypneic and in respiratory distress respond well to NIPPV because it helps to reduce cardiac preload (by increasing intrathoracic pressure) and afterload thus improving forward flow. It also improves atelectasis and gas exchange at the bases of the lungs which can improve hypoxia. NIPPV can reduce the work of breathing, decreasing intrathoracic muscle use, thereby reducing oxygen consumption.
Indications for NIPPV (Canadian Cardiovascular Society):
- High respiratory rate (>25 breaths/min)
- Hypoxia despite high flow oxygen
- Does not recommend routine use of non-invasive ventilation given study (3CPO Trial-see below) demonstrating no difference in mortality, intubation, or admission rates to ICU
Contraindications NIPPV:
- Patients who are not tolerating their secretions
- Patients who are vomiting
- Patients who are unable to protect their airway
Failure of NIPPV (consider endotracheal intubation):
- Failure to improve with 1-2 hours of NIPPV
- Do not tolerate NIPPV
- Contraindications to NIPPV
Tips to help patients tolerate NIPPV:
- The mask – have the patient hold the mask gently on their face to start (rather than strapping it on tightly), coaching the patient to let the mask do the work
- Start pressure low and titrate up:
- C-PAP: begin with pressures of ~6cm H2O, and increase to 12-14 cm H2O
- B-PAP: begin with end expiratory pressures of ~6cm H2O, and inspiratory pressure of ~10cm H2O and slowly increase
- Pitfall – cranking NIPPV above 20cm H2O – at about 20cm H2O the esophagus starts to open, increasing the risk of vomiting and aspiration, so keep this window narrow!
- Adjunct medications – consider fentanyl, ketamine, dexmedetomidine if patient is still having difficulty tolerating the mask (keeping in mind that they all have respiratory or cardiac depressant effects)
The Evidence for NIPPV in CHF: Controversy Surrounding the 3CPO Trial
Studies have shown significant mortality benefit (NNT 13) and avoidance of intubation (NNT 8) with the use of NIPPV in acute heart failure. Though not demonstrating a mortality benefit, other studies have shown that in comparison to standard oxygen delivery NIPPV showed a benefit in terms of self-reported dyspnea, tachycardia, acidosis, and hypercapnia, with no treatment related adverse events compared to standard oxygen delivery.
3CPO Trial (2008): A large multi-center RCT of C-PAP and B-PAP in the UK, that looked at 1069 patients in severe cardiogenic pulmonary edema. Patients were randomized to receive standard oxygen, C-PAP and B-PAP. Results demonstrated no significant difference between the 3 arms on the following parameters: 7-day mortality, 30-day mortality, intubation rates, admission to ICU.
They noted earlier resolution of respiratory distress and metabolic acidosis in the C-PAP/B-PAP arm. It should be recognized that patients who failed standard oxygen therapy were able to use NIPPV. This means that the sickest patients in the standard arm may have received the intervention but were still counted in the standard arm.
In a subsequent meta-analysis of the use of NIPPV in acute cardiogenic pulmonary edema that included the 3CPO, the evidence gathered supported the use of NIPPV for patients in acute pulmonary edema. They were unable to detect a significant difference between C-PAP and B-PAP when they were compared directly. They found that NIPPV reduced mortality, reduced the need for intubation and ICU admission in patients who fail high flow oxygen, and improved the patient’s subjective symptoms.
Bottom Line: Despite controversy stemming from 3CPO trial, the best evidence to date suggests that NIPPV reduces mortality, need for intubation and ICU admission rates, but only in patients who fail high flow oxygen.
What is the role of high flow nasal cannula (HFNC) in the management of acute heart failure?
A small observational study in 2019 compared HFNC to intubation in patients with acute CHF and found that they had similar outcomes. 87% of patients who were in the HFNC group recovered from progressive hypoxemia without the need for endotracheal intubation. HFNC acts to decrease physiologic dead space in the upper airway, increase the delivery of FiO2 and may provide a small amount of PEEP. It should be considered in patients who are not tolerating B-PAP/C-PAP to stave off intubation. However, it is not recommended as a first line oxygenation strategy in acute heart failure patients who fail a nonrebreather.
Bottom Line: in those patients who are failing a nonrebreather, NIPPV is considered first line, and if it fails, consider HFNC to prevent the need for endotracheal intubation.
Nitroglycerin is the first line medication in acute heart failure without cardiogenic shock
Nitroglycerin is the first line medication in patients with acute heart failure who are not in cardiogenic shock as it reduced afterload and preload and redistributes fluid from the pulmonary system rapidly. It has been shown to improve hemodynamic status, respiratory distress, reduces intubation rates and ICU admissions, however it has not been shown to improve mortality. Canadian Cardiovascular Society suggests IV nitroglycerin in patients with SBP >100.
Our experts’ approach to administering nitroglycerin:
Start with 3 sprays or tabs sublingual (400ug x3 =1200ug), apply NIPPV (when indicated), and start a nitroglycerin infusion (50-100 ug/min, and titrate to 100-200ug/min)
Common pitfall: not giving enough nitroglycerin when we start an infusion; remember that 1 spray of nitroglycerin is ~ 400 ug sublingual, so starting a nitro infusion at 5 ug/min akin to a homeopathic dose! Rather, nitroglycerin infusions should be started at 50-100 ug/min depending on the patient’s blood pressure.
Update 2024: An open-label randomized control trial including 54 patients with sympathetic crashing acute pulmonary edema (SCAPE) found that patients receiving high-dose nitroglycerin (600-1000mcg IV bolus followed by 100mcg/min) compared to conventional low-dose nitroglycerin (no bolus, 20-40mcg/min) had higher symptom resolution at 6 hours (65.4% vs 11.5%; P<0.001) and 12 hours (88.5% vs 19.5%; P<0.001), shorter hospital stay (12 hours vs 72 hours), less frequent MACE (3.8% vs 26.9%; P=0.02), and lower intubation rate (3.8% vs 19.2%; P=0.08). Abstract
Suggested protocol for SCAPE management using IV nitroglycerin and NIPPV (source: REBEL EM)
Based on the following protocol, a 2021 study suggests that in patients with SCAPE high dose NTG (600 – 1000mcg) bolus and NTG drip (100ug/min) and NIPPV is a safe strategy that may help to reduce the rate of endotracheal intubation and ICU admission.

Source: REBEL EM adapted from JEM https://rebelem.com/i-love-me-some-high-dose-ntg-and-niv-for-scape/
Which patients with acute heart failure require diuretics in the ED?
Indications for diuretics in acute heart failure: For patients with total body volume overload (eg. history of medication non-compliance and fluid restriction non-compliance with pedal edema, JVD, bibasilar crackles, plump IVC on PoCUS), removal of excess fluid via diuresis is likely to improve perfusion of the heart and kidneys by decreasing venous congestion and improving forward flow (Forward flow = MAP – CVP). Is is important to realize, however, that many patient with acute heart failure do not have true total body volume overload but instead have isolated fluid backed up into the pulmonary system, so diuresis may harm rather than help with renal perfusion. It is therefore important to consider a total body volume assessment in patients who present with acute heart failure.
The timing and dose of furosemide in the management of acute heart failure
On the one hand, diuresis often takes at least 30-60 minutes to occur after administering IV diuretics in the acute heart failure patient supporting the argument that there is no rush to administer diuretics in the ED. On the other hand, there is some theoretical evidence to suggest that the pulmonary vasodilatory effects of furosemide occur within the first 10 minutes of administration.
REALITY-AHF, a prospective, multicenter observational study evaluating door-to-furosemide time in acute heart failure (AHF) (1291 patients, AHF with volume overload) demonstrated that in hospital mortality decreased in those patients who received furosemide within 60 minutes compared to those receiving it > 60 minutes after presentation to the ED. However, this study does have some flaws (see REBEL EM).
IV furosemide dose – our experts suggest 1-2x their total daily home dose of furosemide as an IV bolus
Infusion vs. bolus furosemide?
A metaanalysis of studies comparing intermittent bolus furosemide vs continuous infusion found that “there was no difference between continuous infusion and bolus of furosemide for all-cause mortality, length of hospital stay and electrolyte disturbance, but continuous infusion was superior to bolus administration with regard to diuretic effect and reduction in brain natriuretic peptide.” Infusions are more labour intensive to set up, and may require an ICU admission for the sole purpose of managing the infusion (perhaps poor utilization of resources). Patients who receive infusions of furosemide tend to receive less total amount of drug compared to intermittent bolusing. The ototoxicity associated with furosemide is based on the overall volume and the speed at which it is administered. So, for patients needing high doses of furosemide infusions may reduce the risk of ototoxicity.
Furosemide in patients with renal insufficiency – a challenging subset of patients
In patients with chronic renal failure who are taking an effective dose of diuretics at home, consider 2x their total daily oral dose as an IV bolus trial. If diuresis does not occur within an hour, consider repeating the dose, taking into consideration that excessive use of furosemide may worsen their renal function and/or lead to electrolyte abnormalities. It is reasonable to repeat the creatinine and electrolytes in the ED after administering high dose diuretics to patients with a history of renal insufficiency and balance the risk of worsening renal insufficiency and electrolyte abnormalities with the benefits of diuresis.
Patients with acute heart failure, fluid overload and renal failure who are not responding to furosemide are patients who are likely going to be admitted to the ICU, and potentially receive dialysis. Second line diuretics such as oral matalazone or IV chlorathizide may be considered for those patients who fail furosemide, however it is reasonable to leave the decision on second line diuretics to the admitting team.
Is there a role for morphine in acute heart failure? Choice of anxiolytic
Morphine used to be a staple in treating acute heart failure. However, the ADHERE analysis suggests that in high doses it is associated with worse outcomes (higher mortality, intubation rates, ICU admission rates). Our experts recommend that in those patients in whom you believe anxiety is contributing to their work of breathing and/or who are having difficulty tolerating NIPPV, to consider fentanyl as an anxiolytic as it has a short half-life and is relatively cardiac stable compared to morphine. Other medications you might consider in this context are midazolam (especially in patients with cocaine induced heart failure), ketamine or dexmedetomidine.
Is there a role for ACE inhibitors or ARBs in acute heart failure?
ACE inhibitors/ARBS may reduce afterload in patients who are stabilized, however early administration of these therapies in patients who are unstable increases the risk of kidney injury and hypotension. It is therefore not recommended by our experts to start an ACEi/ARB in the ED, and rather delay until the patient is hemodynamically stable and does not require any further diuresis.
Pitfall: administering medications that decrease afterload in patients with severe aortic stenosis may precipitously drop their blood pressure; in patients with a known history of severe aortic stenosis or a new systolic aortic murmur it is best to avoid the use of nitroglycerin and ACEi/ARBs.
Take home points for management of acute heart failure
- Consider the use of PoCUS, not only for B-lines, but for JVD, cardiac contractility and IVC collapsibility; it can help you out with the diagnosis and the underlying cause.
- Divide these patients into those with cardiogenic shock and those without; those without cardiogenic shock are usually have a subacute presentation with gradual volume overload, but a minority will present dramatically with SCAPE, which requires aggressive timely management
- The goals of management can be summarized with PPV HAVoC
- PPV – start with gentle application to the face at 6cm of water with or without some fentanyl, titrate up to 12-ish and when you’ve hit a decent target, titrate back down. If they can’t tolerate NIPPV, try high flow nasal cannula, which may prevent the need for intubation.
- Hypotension correction with vasopressors – we’ll get to the details in part 2.
- Afterload reduction with nitroglycerin – start with 3 sublingual sprays until an IV infusion is set up and don’t be wimpy with the dosing, avoid high dose morphine, and if you need some anxiolysis, give small doses of fentanyl which may reduce afterload too. Leave ACEi for the admitting team, and avoid afterload reduction in those with severe aortic stenosis or new systolic aortic murmur.
- Volume status – if you’re going to give furosemide based on total body overload gleaned from a volume status assessment, probably best to give it early, be particular about dosing and monitor creatinine and electrolytes carefully in those with renal insufficiency; you can consider adding a second diuretic if you’re getting nowhere – discuss it with the admitting team.
- Cause – treat the underlying cause – very important – is it ischemia? Is it a blown valve? Is it tamponade? Is it myocarditis? Is it a high flow state like thyrotoxicosis or severe anemia? Is it COPD?
In Part 2 we discuss the management of the patient with acute heart failure and cardiogenic shock.
See Episode 4: Acute Congestive Heart Failure for an overview on acute heart failure
Go to emcasessummit.com for streaming package that includes Dr. Hedayati’s brilliant talk on management of heart failure.
Go to part 2 of this 2-part podcast on acute heart failure
References
- Rational Clinical Exam Series: Wang CS, et al. Does This Dyspneic Patient in the Emergency Department Have Congestive Heart Failure? JAMA 2005:294(15);1944-56
- Nakao S, Vaillancourt C, Taljaard M, Nemnom MJ, Woo MY, Stiell IG. Diagnostic Accuracy of Lung Point-Of-Care Ultrasonography for Acute Heart Failure Compared With Chest X-Ray Study Among Dyspneic Older Patients in the Emergency Department. J Emerg Med. 2021 Aug;61(2):161-168. doi: 10.1016/j.jemermed.2021.02.019. Epub 2021 Mar 29.
- Avila, J., Kim, D. (2015, December 18). US against the World: Ultrasound in differentiating COPD from CHF. CanadiEM. Retrieved October 4, 2021, from https://canadiem.org/us-world-ultrasound-differentiating-copd-chf/.
- Martindale JL and coll., Diagnosing Acute Heart Failure in the Emergency Department: A Systematic Review and Meta-analysis. Acad Emer Med 2016;23:223–242.
- Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004;100(1):9-15.
- Al Deeb M and coll., Point-of-care ultrasonography for the Diagnosis of Acute Cariogenic Pulmonary Edema in Patients Presenting with Acute Dyspnea: A systematic Review and Meta-Analysis. Acad Emer Med 2014;21:844–852.
- Ezekowitz, J. A., O’Meara, E., McDonald, M. A., Abrams, H., Chan, M., Ducharme, A., Giannetti, N., Grzeslo, A., Hamilton, P. G., Heckman, G. A., Howlett, J. G., Koshman, S. L., Lepage, S., McKelvie, R. S., Moe, G. W., Rajda, M., Swiggum, E., Virani, S. A., Zieroth, S., Sussex, B. (2017). 2017 comprehensive update of the Canadian Cardiovascular Society Guidelines for the management of heart failure. Canadian Journal of Cardiology, 33(11), 1342–1433. https://doi.org/10.1016/j.cjca.2017.08.022
- Farkas, J. (2021, August 10). Cardiogenic Shock & Severe CHF. EMCrit Project. Retrieved October 4, 2021, from https://emcrit.org/ibcc/chf/.
- Gray, A. (2008). Non-invasive ventilation in acute cardiogenic pulmonary edema. New England Journal of Medicine, 359: 142-151. DOI: 10.1056/NEJMoa0707992
- Marik PE, Flemmer M. Narrative review: the management of acute decompensated heart failure. J Intensive Care Med 2012; 27: 343-53.
- Vital FMR, Saconato H, Ladeira MT, et al. Non-invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary edema. Cochrane Database of Systematic Reviews 2008, Issue 3. No.: CD005351.
- Cui-Lian Weng, Yun-Tao Zhao, Qing-Hua Liu, et al. Meta-analysis: Noninvasive Ventilation in Acute Cardiogenic Pulmonary Edema. Ann Intern Med.2010;152:590-600. [Epub ahead of print 4 May 2010].
- Kang, MG et al. Clinical efficacy of high-flow oxygen therapy through nasal cannula in patients with acute heart failure. J Thorac Dis. 2019 Feb;11(2):410-417.
- Salim Rezaie, “Door to Furosemide (D2F) in Acute CHF…Really?”, REBEL EM blog, November 27, 2017. Available at: https://rebelem.com/door-to-furosemide-d2f-in-acute-chfreally/.
- Matsue Y et al. Time-to-Furosemide Treatment and Mortality in Patients Hospitalized With Acute Heart Failure. JACC 2017. PMID: 28641794
- Kamperidis, V., Delgado, V., van Mieghem, N.M., Kappetein, A.-P., Leon, M.B. and Bax, J.J. (2016), Diagnosis and management of aortic valve stenosis in patients with heart failure. Eur J Heart Fail, 18: 469-481.
- Berbenetz N, Wang Y, Brown J, et al. Non-invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary oedema. Cochrane Database Syst Rev. 2019;4(4):CD005351.
- Mathew R, Kumar A, Sahu A, Wali S, Aggarwal P. High-Dose Nitroglycerin Bolus for Sympathetic Crashing Acute Pulmonary Edema: A Prospective Observational Pilot Study. J Emerg Med. 2021 Sep;61(3):271-277.
- Ng KT, Yap JLL. Continuous infusion vs. intermittent bolus injection of furosemide in acute decompensated heart failure: systematic review and meta-analysis of randomised controlled trials. Anaesthesia. 2018 Feb;73(2):238-247
- Peacock WF, Hollander JE, Diercks DB, Lopatin M, Fonarow G, Emerman CL. Morphine and outcomes in acute decompensated heart failure: an ADHERE analysis. Emerg Med J. 2008 Apr;25(4):205-9.
Other FOAMed resources on acute heart failure
EMdocs on management pearls and pitfalls
REBEL EM on morphine in heart failure
REBEL EM on nitroglycerin for SCAPE
Internet book of critical care on SCAPE
EMdocs on furosemide in pulmonary edema
Drs. Helman, Hedayati and Tillmann have no conflicts of interest to declare
Now test your knowledge with a quiz.




It is good to listen to the lungs for wheeze in that there may well be an element of Bronchospasm and salbutamol ought help . Used to be cardiac asthma . Low dose morphine 1-2.5mg intravenously is still recommended by the Cardiovascular Australian Therapeutic Guidelines 2018 P 132 if there is distress .
Thank you for another great podcast!
I noticed you didn’t mention thoracostomy / draining pleural fluid / pleurosentesis in the “warm and wet” AHF patient. This is a topic that confuses me , and it seems there’s high variability as to whether it is performed and when it is performed . At our hospital we do a lot of these and I’ve never really understood why
In 2016 the ESC (European society of cardiology) wrote “ In patients with AHF and pleural effusion, pleurocentesis with fluid evacuation may be considered if feasible in order to alleviate dyspnoea”
But it has fallen out of the 2021 guideline , without (to me at least) any argument for why it has been removed
Certain experts recommend it (ie UpToDate): “Drainage for symptomatic patients — Most experts agree that patients with a symptomatic NMPE, should undergo a drainage procedure”
“Asymptomatic patients with NMPE do not typically require therapeutic pleural intervention”
but articles I’ve managed to find on the topic seem not to encourage this
“ Of those patients with decompensated heart failure requiring diuretic treatment, 87% have pleural effusions on CT [21]. Patients with uncomplicated heart failure with pleural effusions have bilateral effusions in 73% of cases [22]. A large proportion of these pleural effusions will improve with optimised treatment for heart failure. A prospective study of 60 patients demonstrated that 89% of those patients with an initial response to diuretic treatment no longer had a pleural effusion after 2 weeks of follow-up”
Bintcliffe et al, 2016: The management of benign non-infective pleural effusions
And
One source seems retrospectively (not necessarily causality) to find worse outcome in patient cohort who had pleurosentesis performed as opposed to a matched control group
Sherry et al 2020: Outcomes of Thoracentesis for Acute Heart Failure in Hospitals
My bottom line is: should almost never be performed in AHF in the ED , as it probably is mainly symptom relief and with the current lack of studies this might as well be placebo.
But I’m curious to what your practise is / what you guys think on this topic ?
All the best
Peter , EM resident
I listened again in March 2022 and heard that it is hard to apply Positive Pressure Ventilation with that Tight Mask and the air blowing in .
But what about IV frusemide stay it actually starts to work in minutes though not peaking till half an hour .
Or Nitro as a drip.or sublingually.
Why drop frusemide completely suddenly .
For Primary Care house calls with Oxygen worked time and time well BUT the dose had to be precise .
HF excacerbation due to infection with a concern of sepsis what do we do then? Fluids? diuretics?
I’m still very uncomfortable about that.
Thanks a lot for another great apisode,
Itay, med student
Dr Anton Helman
Dear sir
Greatly appreciate your effort ,bringing us the latest knowledge in a palatable way,to keep us updated and prepared in our work.