In this ECG Cases blog we look at 5 patients with potentially ischemic symptoms and LBBB. Which required cath lab activation?
Written by Jesse McLaren; Peer Reviewed and edited by Anton Helman, July 2020.
Five patients presented with potentially ischemic symptoms. Which required cath lab activation?
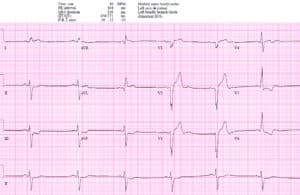
Patient 1: 90y/o history CAD, old LBBB with VT arrest, cardioverted by EMS
Patient 2: 65y/o with bilateral shoulder pain. AVSS. Old then new ECG
Patient 3: 70y/o with one week intermittent chest pain now constant. AVSS.
Patient 4: 60y/o new onset chest pain. AVSS
Patient 5: 50y/o dialysis patient with 24 hours chest pain, AVSS, trop 1500.
LBBB + occlusion MI
The left bundle branch provides rapid conduction via anterior and posterior fascicles. If it is blocked, conduction remains rapid through the right ventricle but is delayed across the left ventricle. So depolarization is prolonged (QRS>120) and leftward: anterior leads have deep/broad S waves, and lateral leads have slurred or notched R waves. In addition, the initial septal depolarization is reversed and spreads from right to left, resulting in small/no R waves in anterior leads and disappearance of “septal Q waves” in the lateral leads. Like other forms of abnormal depolarization LBBB produces repolarization abnormalities, and the discordant ST elevation and ST depression have caused a dilemma for the STEMI paradigm.
As a review summarized, “not only is the ECG diagnosis of AMI difficult due to ‘masking’ of characteristic ECG changes by altered ventricular depolarization, but these patients may be at higher risk for AMI, congestive heart failure, and death compared with patients without BBB.”[1] The first guidelines treated all patients with ischemic symptoms and LBBB as STEMI, based on early trials. Then the guidelines shifted consider “new LBBB” as STEMI equivalents, based on the assumption it takes a large anterior infarct to block both left fascicles. But most LBBB are not caused by acute ischemia but by chronic heart disease, which is a risk factor for ACS but not an automatic indication for reperfusion therapy. Patients presenting to the ED with ischemic symptoms have similar rates of MI whether they have new LBBB, old LBBB or no LBBB [2]. Treating “new LBBB” as STEMIs led to unnecessary interventions, so this indication was removed from the 2013 AHA guidelines. But this leads to the opposite problem: “the guidelines fail to recognize that some patients with suspected ischemia and LBBB do have STEMI, and denying reperfusion therapy could be fatal.”[3]
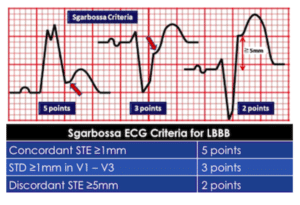
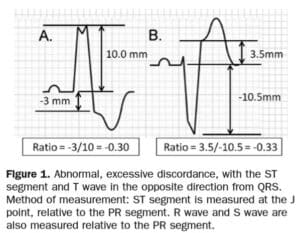
Sgarbossa first identified criteria that can identify AMI in the presence of LBBB. Since LBBB normally produces discordant STE and STD, acute ischemia can be identified by concordant STE, concordant STD anteriorly, or disproportionately discordant STE >5mm[4]:
STEMI describes ST elevation in two contiguous leads, but Sgarbossa identified criteria for acute reperfusion that included concordant STE elevation or depression in one lead. But the criteria was based on enzyme-diagnosed AMI, had a complicated scoring system, and had inadequate sensitivity and specificity (in part because the criteria for disproportionate STE was based on an absolute value regardless of the size of the preceding QRS). Smith studied angiographically-confirmed culprit lesions and identified ECG criteria that can predict occlusion MI, shifting away from the STEMI paradigm and adding diagnostic accuracy to the Sgarbossa criteria. The Smith-modified Sgarbossa criteria replaced the absolute discordance of >5mm with a relative discordance of ST/S<-0.25 (i.e. amplitude of STE greater than 25% of the amplitude of the preceding S wave), and also included any excessive discordance (either STE >30% the preceding S wave, or STD>30% preceding R wave ) in any lead [5].
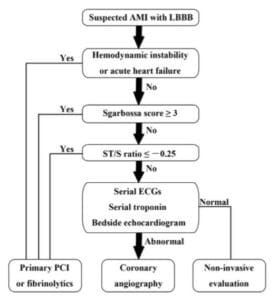
The shift from STEMI to OMI (occlusion MI) includes examining ST changes (both elevation and depression) relative to the preceding QRS and in context of the patient’s symptoms. As the validation study discovered, “we found that the modified Sgarbossa criteria performed similarly well using a proportionality cutoff of -0.20 or -0.25. As expected, however, the cutoff of -0.20 produced slightly higher sensitivity than the cutoff of -0.25 (84% vs 80%) and slightly lower specificity (94% vs 99%). Thus, the decision to use -0.20 or -0.25 as the proportional discordance is not absolute but rather depends on the physician’s pretest probability and clinical context. The ECG should always be used in clinical context, as diagnostic ST elevation is frequently absent in ACO even in normal conduction (ie, no bundle branch block).”[6] Cai (cited below) proposed an algorithm that takes into account the Sgarbossa/Smith criteria, but that starts with the patient’s clinical status. As with refractory ischemia or hemodynamically unstable patients with normal conduction, the 2017 European guidelines emphasize that “patients with a clinical suspicion of ongoing myocardial ischaemia and LBBB should be managed in a way similar to STEMI patients, regardless of whether the LBBB is previously known…Suspicion of ongoing myocardial ischaemia is an indication for a primary PCI strategy even in patients without diagnostic ST-segment elevation.”[7]
Back to the cases
Patient 1: appropriate cath lab activation but no occlusion MI
AF with LBBB, no Sgarbossa/Smith criteria but high-pretest probability patient with hemodynamic instability. Cath lab activated: no acute coronary occlusion.
Patient 2: “new LBBB” but unnecessary cath lab activation
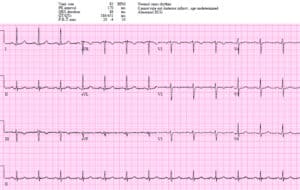
Borderline sinus tachycardia with new LBBB in stable patient with no Sgarbossa/Smith criteria. Unnecessary cath lab activation, no occlusion and negative trop.
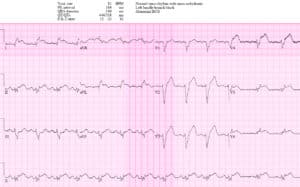
Patient 3: delayed cath lab activation
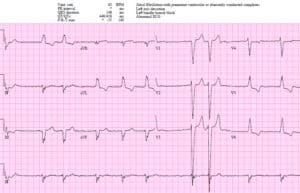
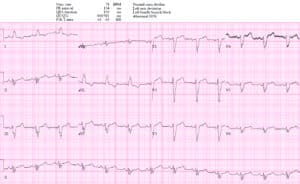
Sinus bradycardia with LBBB in hemodynamically stable patient with ongoing chest pain. Concordant STE in I/aVL, concordant STD in II/III/AVF. Missed by two physicians and cath lab activated after trop came back positive. Second diagonal occlusion. After stent, resolving concordant changes:
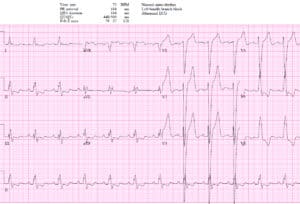
Patient 4: appropriate rapid cath lab activation
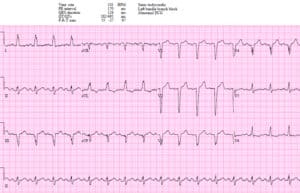
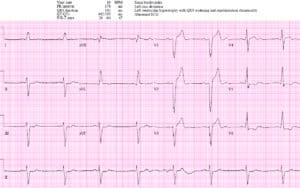
NSR with LBBB in hemodynamically stable patient with ongoing chest pain. Concordant STE I/aVL/V5-6 and II, and excessive discordance in V2 (STE/S=3/15=0.2), V3 (3/10=0.3), and V4(3/5=0.6). EMS direct to cath lab: proximal LAD occlusion wrapping around the apex. Discharge ECG had resolution of Sgarbossa/Smith changes:
Patient 5: cath lab appropriately activated but for the wrong reasons
NSR with LBBB interpreted by cardiology as “positive Sgarbossa criteria with >5mm STE in V2-3” (which would indicate likely LAD occlusion). But the STE in V2-3 is proportional to the massive QRS complexes: STE/S in V2=6/43=0.14, V3=6/53=0.11. There are no modified-Sgarbossa criteria for occlusion MI, but patient had ongoing chest pain with a positive trop requiring cath lab activation, which found a circumflex occlusion (which doesn’t meet STEMI criteria in a quarter of the cases even in patients without LBBB).
Take home points for LBBB + Occlusion MI
- Use the Smith-modified Sgarbossa criteria (concordant STE/STD, or excessively discordant STE/S >25% or STD/R>30%) to identify occlusion MI in the patient with ischemic symptoms and LBBB (whether new or old)
- Treat the patient: those with refractory ischemia and hemodynamic instability from suspected occlusion MI require cath lab activation regardless of the ECG
References for ECG Cases 11: LBBB + Occlusion MI
- Neeland IJ, Kontos MC and de Lemos JA. Evolving considerations in the management of patients with left bundle branch block and suspected myocardial infarction. J Am Coll Cardiol 2012 Jul 10;60(2):96-105
- Chang AM, Shofer FS, Tabas JA, et al. Lack of association between left bundle-branch block and acute myocardial infarction in symptomatic ED patients. Am J of Emerg Med 2009;27:916-921
- Cai Q, Mehta N, Sgarbossa E et al. The left bundle-branch block puzzle in the 2013 ST-elevation myocardial infarction guideline: from falsely declaring emergency to denying reperfusion in a high-risk population. Are the Sgarbossa criteria ready for prime time? Am Heart J 2013 Sep;166(3):409-13
- Sgarbossa EB, Pinkski SL, Barbagelata A, et al. Electrocardiographic diagnosis of evolving acute myocardial infarction in the presence of left bundle-branch block. N Engl J Med 1996;334:481-487
- Smith SW, Dodd KW, Henry TD et al. Diagnosis of ST-elevation myocardial infarction in the presence of left bundle branch block with the ST-elevation to S-wave ration in a modified Sgarbossa rule. Ann Emerg Med 2012 Dec;60(6):766-776
- Meyers HP, Limkakeng AT, Jaffa EJ, et al. Validation of the modified Sgarbossa criteria for acute coronary occlusion in the setting of left bundle branch block: a retrospective case-control study. Am Heart J 2015 Dec;170(6):1255-64
- Ibanez B, James S, Agewall S. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2017 Jan;39(2):1119-177



Leave A Comment