In this ECG Cases blog we look at 8 patients who presented with potential ACS symptoms, and review ECG changes in Spontaneous Coronary Artery Dissection (SCAD)
Written by Jesse McLaren; Peer Reviewed and edited by Anton Helman. February 2023
8 patients with few cardiac risk factors presented with potential ACS symptoms. How would you manage them based on their symptoms and ECG?
Case 1: 55 year old healthy female with 2 days of intermittent chest pain now constant for 4 hours
Case 2: 60 year old healthy female under recent stress, with 3 hours of chest pain radiating to the arm
Case 3: 65 year old healthy female with 2 hours of ongoing chest pain that began while exercising
Case 4: 60 year old female, recent non-STEMI from SCAD treated medically, with recurring chest pain/reflux now resolved. ECG from prior angiogram and new ECG
Case 5: 40 year old healthy female with intermittent chest pain and nausea now resolved
Case 6: 50 year old female with history of hypertension, with 2 days of intermittent chest pain now constant
Case 7: 25 year old male, recent cocaine use, with exertional chest pain that has now resolved
Case 8: 60 year old healthy female with 2 weeks exertional chest pain that resolves with rest. ECG from first visit with normal troponin levels, then ECG from return visit
Approach to SCAD: consider patients and ECG changes
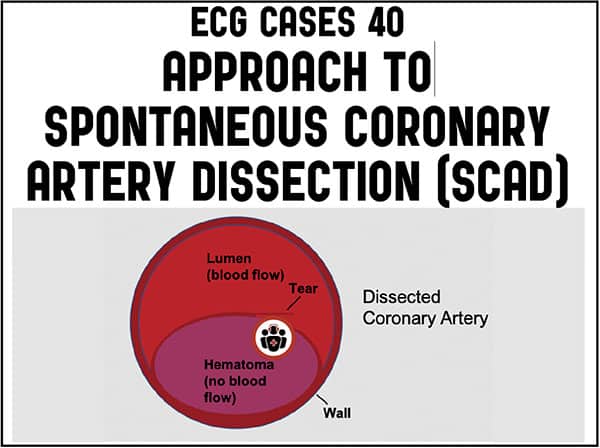
Spontaneous coronary artery dissection (SCAD) was first described in 1931 in a healthy 42 year old female with sudden cardiac death after vomiting.[1] But it is only recently that SCAD has been more widely recognized as a potential cause of Acute Coronary Syndrome, thanks to advances in angiography and advocacy by SCAD patients and providers like the SCAD Alliance. While SCAD makes up only only 3% of all ACS, it causes up to 30% in female patients younger than age 50, and is the most common cause of pregnancy-associated MI. SCAD is separation of the layers of the coronary artery walls by an intramural hematoma, which is spontaneous (i.e. not caused by atherosclerosis, trauma, or iatrogenesis) and may be associated with an intimal tear. The expanding hematoma compresses the true lumen, from narrowing to total occlusion, leading to myocardial ischemia or infarction.[2]
Risk factors for SCAD include 1) predisposing conditions include female sex hormones, conditions that weaken coronary artery walls (especially fibromuscular dysplasia, but also connective tissue or systemic inflammatory conditions), and SCAD patients have higher rates of migraines; and 2) precipitating events that increase shear stress (including sympathetic surge from emotional/physical stress or sympathomimetic drugs, or valsalva). Peripartum SCAD risk factors include high levels of progesterone that weakens the wall, estrogen that creates a hypercoagulable state, the hemodynamic stress of pregnancy, and valsalva during delivery. SCAD has also been reported in cis male patients (often with history of hypertension, migraines, or fibromuscular dysplasia, presenting after physical or emotion stress) and trans female patients taking gender affirming hormone therapy. [3,4]
Even with angiography the diagnosis of SCAD is challenging because angiograms only show the lumen (so the appearance can mimic vasospasm, plaque rupture, or normal vessel narrowing) and advanced imaging like intravascular ultrasound is often required. Intervention is also difficult because the same predisposing factors and pathology create a high risk for iatrogenic dissection. But SCAD usually heals spontaneously so management is usually conservative, unless there is ongoing ischemia or hemodynamic instability. Medical treatment includes continuing dual anti-platelet therapy, discontinuing heparin and avoiding thrombolytics, providing beta-blockers to reduce shear stress, and screening for fibromuscular dysplasia.[5]
In the Canadian cohort study of 750 patients by SCAD expert Dr. Jacqueline Saw, mean age was 51 years with a range from 24 to 89. Women were 88.5% of patients, of whom 55% were post-menopausal. For baseline characteristics, a third had no atherosclerotic risk factors but a third had hypertension and a third had migraines (which are more common in SCAD patients). For predisposing conditions, half had no SCAD risk factors, but 30% had fibromuscular dysplasia, 10% were on hormone therapy, 5% had previous SCAD, and 5% were peripartum. For precipitating events, half had emotional stress and a quarter had physical stress while a third had no clear trigger. More than 90% presented with chest pain and nearly all developed elevated troponin, with a third admitted as STEMI and two thirds as Non-STEMI (and only 0.4% as unstable angina). Approximately 85% were treated conservatively and 9% had in-hospital adverse events (with higher rates for peri-partum SCAD), most of which were recurring MI from SCAD extension or iatrogenic dissection.[6] A three-year follow-up found a 10% rate of recurring MI (most from extension of previous SCAD or de novo recurring SCAD), and mortality rate of less than 1%.[7]
Because SCAD requires angiographic confirmation we will not make this diagnosis in the emergency department, and will not even be aware we saw a case of SCAD unless we follow up on the angiograms of patients admitted as STEMI or Non-STEMI. But there are two ways that we can improve diagnosis and management in the ED, both for SCAD and for ACS in general.
- Patient: consider SCAD (and ACS in general), in younger female patients without atherosclerotic risk factors presenting with ACS symptoms
SCAD is underdiagnosed and undertreated because of who it affects: younger female patients without traditional “cardiac” (i.e. atherosclerotic) risk factors, and this magnifies the existing gender gaps in the diagnosis and treatment of ACS.[8] As an analysis of patients with SCAD in the emergency department concluded, “Missed or delayed diagnosis is common. As an example, there are numerous cases reported where patients with SCAD did not receive a full cardiac evaluation on presentation despite reporting potential ACS symptoms or did not trigger the standard response to elevated biomarkers, leading to significant comorbidity or death. With the underdiagnosis and misdiagnosis of SCAD, in addition to poor outcomes reported in women who present with cardiac events in general, it is important to address the implicit bias likely underlying this patient population to ensure the appropriate diagnostic testing of those ‘atypical’ patients who present with chest pain.”[9] This is compounded by relying on risk stratification tools for atherosclerotic ACS that were not designed for SCAD, or anchoring on an initially normal troponin (which can miss SCAD and STEMI/non-STEMI). But the vast majority of patients with SCAD present with chest pain, troponin nearly always becomes elevated, and the majority of ECGs show ischemic changes. So the first step is simply to consider/not dismiss ACS in younger female patients without atherosclerotic risk factors who present with potential ACS symptoms, and to include SCAD on the differential of peripartum patients with chest pain.[10]
- ECG: Occlusion MI, reperfusion, or non-occlusive MI
Like takotsubo cardiomyopathy or myocarditis, SCAD is an angiographic diagnosis of exclusion, because type 1 MI from atherosclerotic plaque rupture is a far more likely cause of ACS (including in younger women) and has more time-sensitive treatment. The ECG in ACS is usually dichotomized into STEMI vs non-STEMI, and most literature on SCAD replicates these general descriptions of ST elevation, ST depression, T wave inversion, or normal. But STEMI criteria have high false positive and false negative rates, so ST elevation can be non-ischemic or a coronary artery can be completely occluded without the ECG ever meeting STEMI criteria. STEMI criteria also don’t account for the dynamic process of spontaneous reperfusion and reocclusion that are also possible in SCAD. For example, in the Canadian cohort study the angiographic TIMI flow was reduced to 0-2 in a third (including 9% with complete occlusion, TIMI 0), while two-thirds had normal TIMI 3 flow. So SCAD can result in both occlusive and non-occlusive MI, and the dynamic nature of SCAD (including dissection extension, dynamic compression, and potential collateral circulation), could result in occlusions spontaneously reperfusing and reoccluding.
From an emergency perspective, undifferentiated ACS patients are best categorized not as STEMI vs Non-STEMI, but Occlusion MI requiring immediate reperfusion (whether they meet STEMI criteria or not), spontaneous reperfusion at risk of reocclusion, and Non-Occlusive MI that can wait for non-urgent angiogram.[11] We can also use this framework for ACS patients who may or may not have SCAD, both to reduce delayed reperfusion for OMI and delayed diagnosis of SCAD (with resulting delayed discontinuation of heparin):
- STEMI(+)OMI (ie. ischemic symptoms and ECG with true positive STEMI): need and will likely get rapid cath lab activation, with reperfusion of OMI (and diagnosis/management of SCAD, with possible intervention for ongoing ischemia)
- STEMI(-)OMI (i.e ischemic symptoms and ECG that doesn’t meet STEMI criteria but is diagnostic of Occlusion MI, clinical OMI with refractory ischemia without ECG changes): need cath lab activation but at at risk for delays because of STEMI paradigm, resulting in delayed reperfusion of OMI (and delayed diagnosis/management of occlusive SCAD, with possible intervention for ongoing ischemia, and discontinuatio of heparin)
- OMI with spontaneous reperfusion (i.e. resolved ischemic symptoms with ECG signs of reperfusion): needs urgent angiogram but risk of delays with risk of spontaneous reocclusion (including secondary to SCAD, though reperfused SCAD will unlikely get intervention)
- Non-Occlusive MI (i.e. resolved ischemic symptoms, no ECG signs of occlusion or reperfusion): appropriate non-urgent angiogram because artery is open, with small MI from non-occlusive thrombus (or non-occlusive SCAD, which will unlikely get intervention)
Back to the cases
Case 1: inferoposterolateral STEMI(+)OMI secondary to SCAD
- Heart rate/rhythm: normal sinus
- Electrical conduction: normal
- Axis: normal
- R-wave progression: late
- Tall/small voltages: normal voltages
- ST/T changes: inferior ST elevation wiht reciprocal ST depression I/aVL, anterior ST depression, and lateral ST elevation
Impression: inferoposterolateral STEMI(+)OMI. Cath lab activated: right coronary artery 100% occluded from SCAD, EF 45% wiht apical/infero/lateral akinesis. Because of ongoing ischemia and size of infarct, PCI attempt was made but unsuccessful, so treated medically. First troponin I was 500 ng/L (normal <16 in females and <26 in males) and peak 15,000. Discharge ECG showed inferior Q waves and reperfusion T wave inversion:
Case 2: first diagonal STEMI(+)OMI secondary to SCAD
- H: borderline sinus tach
- E: RBBB
- A: normal axis
- R: normal R wave progression
- T: normal voltages
- S: convex ST elevation and hyperacute T waves I/aVL/V2 wiht inferior reciprocal change, and lateral ST depression
Impression: first diagonal STEMI(+)OMI with subendocardial ischemia. Cath lab activated: first diagonal 95% occluded by SCAD with TIMI 2 flow, treated with balloon angioplasty because of occlusion and ongoing chest pain. First troponin 100 and peak 20,000. Discharge ECG had Q wave and reperfusion T wave in version I/aVL/V2, with reciprocally tall T waves inferiorly, and resolution of lateral subendocardial ischemia:
Case 3: first diagonal STEMI(-)OMI secondary to SCAD
- H: normal sinus rhythm
- E: normal conduction
- A: normal axis
- R: fragmentted QRS in V2
- T: normal voltages
- S: mild ST elevation in aVL and hyperacute T wave in V2, with reciprocal inferior ST depression
Impression: first diagonal STEMI(-)OMI with ECG mislabeled “normal”. Cath lab activated: first diagonal occlusion from SCAD, treated medically. First troponin 60 and peak 22,000, with apical hypokinesis on echo. Discharge ECG showed anterolateral reperfusion T wave inversion:
Case 4: LAD reperfusion, secondary to SCAD
- H: normal sinus rhythm
- E: normal conduction
- A: physiologic left axis
- R: loss of R wave progression
- T: normal voltages
- S: first ECG had subtle anterior ST elevation and hyperacute T waves. Second had primary T wave inversion in antero-inferior distribution
Impression: subtle distal LAD occlusion (first ECG) then reperfusion (second ECG). Serial troponin normal, so ECG likely represented reperfusion from the first visit. Admitted as unstable angina with repeat angiogram showing normal flow in LAD. Symptoms attributed to SCAD vs coronary vasospasm, treated medically.
Case 5: subtle RCA reperfusion, secondary to SCAD
- H: normal sinus rhythm
- E: normal conduction
- A: normal axis
- R: early R wave progression
- T: normal voltages
- S: mild T wave inversion III/aVF and V3
Impression: early R wave progression and inferior T wave inversion suggesting possible inferoposterior reperfusion. Patient was painfre, trop was 1500 and admitted as Non-STEMI. Angiogram showed right coronary artery SCAD with collaterals, treated medically. Screening CT also found celiac artery aneurysm from fibromuscular dysplasia.
Case 6: silent circumflex occlusion secondary to SCAD
- H: normal sinus rhythm
- E: normal conduction
- A: normal axis
- R: normal R wave progression
- T: normal voltages
- S: nonspecific T wave flattening
Impression: nondiagnostic ECG but ongoing ischemic symptoms. Troponin rose from 1,800 to 4,000 and admitted for Non-STEMI. Initial angiogram appeared normal and was diagnosed as Myocardial Infarction with Nonobstructive Coronary Arteries (MINOCA), but cardiac MRI showed focal lateral infarction, and review of angiogram suggested circumflex SCAD. Was treated medically and discharge ECG showed lateral reperfusion T wave inversion:
Case 7: early repolarization and lead misplacement with pseudo-STEMI, elevated troponin from non-occlusive SCAD
- H: sinus arrhythmia but P wave larger in I than in II
- E: normal conduction
- A: left axis deviation with inverted P/QRS/T in III
- R: normal R wave progression
- T: tall voltages with J waves
- S: proportional ST segments and T waves
Impression: left arm/left leg arm reversal and early repolarization, with pseudo-lateral STEMI. ECG repeated with correct lead placement:
Still flagged by computer as ST elevation, but this is normal variant in 25 year old with large voltages, early repolarization and proportional ST/T waves. Patient remained painfree. Troponin rose from 500 to 3,000 and coronary CT revealed 25-50% LAD stenosis with intramural hematoma, i.e. non-occlusive MI secondary to SCAD. Treated with aspirin and discharge ECG was unchanged.
Case 8: from unstable angina to non-occlusive MI from circumflex SCAD with collaterals
- H: normal sinus rhythm
- E: normal conduction
- A: left axis from LAFB
- R: delayed R wave from LAFB
- T: normal voltages
- S: no ST/T changes
Impression: non-diagnostic ECGs. Troponin on second visit rose from 300 to 3,000. Angiogram revealed tortuous vessels with the obtuse marginal branch of the circumflex artery 99% occluded by SCAD but perfused by collaterals. Screening CT found mesenteric fibromuscular dysplasia. Treated medically and discharge ECG was unchanged.
Take home points on Spontaneous Coronary Artery Dissection (SCAD)
- Consider SCAD (and ACS in general) in younger female patients without atherosclerotic risk factors presenting with ACS symptoms
- Patients with ischemic symptoms and STEMI(+)OMI or STEMI(-)OMI require immediate cath lab to rule out occlusion and diagnose SCAD
- Patients with resolved symptoms and signs of spontaneous reperfusion are at risk for reocclusion
- ACS patients (including SCAD) can have nonischemic ECGs despite silent occlusion (especially circumflex), occlusions perfused by collaterals, or from non-occlusive MI
References for ECG Cases 40 – Approach to Spontaneous Coronary Artery Dissection
- Pretty HC. Dissecting aneurysm of coronary artery in a woman aged 42. BMJ 1931, 1, 667
- Saw J, John Mancini GB, Humphries KH. Contemporary review on Spontaneous Coronary Artery Dissection. J of Am Coll Cardiol 2016 July 19;68(3):297-312
- Fahmy P, Prakash R, Starovoytov A, et al. Pre-disposing and precipitating factors in men with spontaneous coronary artery dissection. J of Am Coll Cardiol 2016 Apr 25;9(8):866-868
- Hirsch K, Yogeswaran V, Dean LS. Spontaneous coronary artery dissection and exogenous estrogen in a transgender female. Cathter Cardiovasc Interv 2022 Jul;100(1):96-99
- Hayes S, Tweet MS, Adlam D, et al. Spontaneous coronary artery dissection: JACC state-of-the-art review. J of Am Coll Cardiol 2020 Aug 25;76(8):961-984
- Saw J, Starovoytov A, Humphries K, et al. Canadian spontaneous coronary artery dissection cohort study: in-hospital and 30-day outcomes. Eur Heart J 2019 Apr 14;40(15):1188-1197
- Saw J, Starovoytov, Aymong E, et al. Canadian Spontaneous Coronary Artery Dissection cohort study: 3-year outcomes. J of Am Coll Cardiol 2022 Oct,80(17):1585-1597
- Chandrasekhar J, Gill A, Mehran R. Acute myocardial infarction in young women: current perspectives. Int J Womens Health 2018;10:267-284
- Johnson AK, Tweet MS, Rouleau SG, et al. The presentation of spontaneous coronary artery dissection in the emergency department: signs and symptoms in an unsuspecting population. Acad Emerg Med 2022 Apr;29(4):423-428
- Lee R, Carr D. Pregnancy-associated spontaneous coronary artery dissecetion (PASCAD): an etiology for chest pain in the young peripartum patient. CJEM 2018:S64-S69
- Meyers HP, Bracey A, Lee D, et al. Comparison of the ST-Elevation Myocardial Infarction (STEMI) vs NSTEMI and Occlusion MI (OMI) vs NOMI paradigms of acute MI. J Emerg Med 2021 Mar;600(3);273-284



















Hi,
Thanks for your hard work on this.
You mention not using thrombolytics in SCAD. If you work in a place far from PCI, I think on balance the benefits of lytics outweigh risks. What are your thoughts?
Thanks,
Tim
Thanks TIm, and I agree. Without angiographic confirmation, patients with STEMI/OMI are far more likely to have an acute thrombotic occlusion than SCAD, and the risks of withholding lytics for the former group is far greater than the risks of thrombolytics in SCAD.