In Part 1 of Pulmonary Embolism Challenges in Diagnosis Drs. Helman, Lang and DeWit discussed a workup algorithm using PERC and Wells score, the bleeding risk of treated pulmonary embolism, pearls in decision making on whether or not to work up a patient for pulmonary embolism, how risk factors contribute to pretest probability, the YEARS criteria and age-adjusted D-dimer. In this Part 2 we answer questions such as: what are the important test characteristics of CTPA we need to understand? Which patients with subsegmental pulmonary embolism should we treat? When should we consider VQ SPECT? What is the best algorithm for the work up of pulmonary embolism in pregnant patients? How best should we implement pulmonary embolism diagnostic decision tools in your ED? and many more…
Podcast production, sound design & editing by Anton Helman, additional editing by Sucheta Sinha
Written Summary and blog post by Shaun Mehta & Alexander Hart, edited by Anton Helman August 2018
Cite this podcast as: Helman, A, Lang, E, DeWit, K. Pulmonary Embolism Challenges in Diagnosis 2 – Imaging, Pregnancy, Subsegmental PE. Emergency Medicine Cases. August, 2018. https://emergencymedicinecases.com/pulmonary-embolism-diagnosis-2-imaging-pregnancy-subsegmental-pe/. Accessed [date].
CTPA test characteristics and pulmonary embolism diagnosis
As with the rest of emergency medicine, our interventions are rarely benign. In order to avoid unnecessary radiation and major bleeding complications as a result of anticoagulating patients with false positive CTPA results, it’s important to have a rational approach to imaging for PEs as well as a good approach to shared decision making with our colleagues, our radiologists and our patients.
Although CTPA has become the gold standard for diagnosing PE and remains the best imaging modality available, it is far from perfect. The CTPA is prone to over-diagnosing clinically irrelevant emboli in low-risk patients [1]. Furthermore, although its sensitivity approaches 100% for clinically relevant PEs, in those with high pre-test possibility there is a small chance a clot might be missed. Those patients at high risk for PE based on a Wells score >6 with a negative CTPA should be counseled that although the present CTPA does not show a PE, up to 5% of high risk patients may develop a PE within a few months of a negative CTPA [2,3].
What about clot burden and location? These imaging characteristics have not been shown to accurately predict outcome, or even symptoms. The clinical context is much more important, and markers such as hypotension and hypoxia are better predictors of outcome [4].
Subsegmental pulmonary embolism: To treat or not to treat?
In the last 10 years, the incidence of diagnosed PE has doubled, despite no change in mortality, partly due to advances in CT technology and partly due to radiologists overcalling subsegmental PEs due to medico-legal concerns. With modern CTs, subsegmental PEs are more often diagnosed. Although there is some variability in practice, most emergency physicians end up treating subsegmental PEs. But should we?
An observational study by Goy et al. in 2015 reviewed 2213 patients with a diagnosis of subsegmental PE, and showed that whether or not anticoagulation was given, there were no recurrent PEs, yet 5% of anticoagulated patients developed life-threatening bleeding [5]. Other studies have yielded similar results [6].
Shared decision-making. Consider the patient’s bleeding risk (HASBLED score) and discuss potential treatment options. The 2018 ACEP Clinical Policy on Acute Venous Thromboembolic Disease gives withholding anticoagulation in patients with subsegmental PE a Level C recommendation and states: “Given the lack of evidence, anticoagulation treatment decisions for patients with subsegmental PE without associated DVT should be guided by individual patient risk profiles and preferences [Consensus recommendation].”
Start anticoagulation for subsegmental PE in the ED with an expectation that anticoagulation may be stopped in follow-up. While the risk of major bleeding with a full course of anticoagulation is significant, the risk of bleeding with a few doses of anticoagulant is very low. Thus, starting treatment for subsegmental PE in the ED and referring the patient for early timely follow up in a thrombosis or internal medicine clinic (within a few days) is a reasonable option. Counseling your patient that the consultant may recommend stopping the anticoagulant is essential to avoid conflicting messages. Consultants may risk stratify low risk patients with serial leg dopplers to direct ongoing therapy.
Update 2022: Multi-center prospective cohort study across 18 sites from 2011-2021 including 292 patients with subsegmental pulmonary embolism (PE) (without proximal deep venous thrombosis), managed without anticoagulation. Found recurrent venous thromboembolism in 3.1% of patients during 90-day follow-up period, with no fatal recurrent PE. Excluded patients who were hospitalized, pregnant, had active cancer, history of PE/DVT or hypoxia. Abstract
V/Q Scan in pulmonary embolism challenges in diagnosis
Many emergency physicians are comfortable using D-dimers, dopplers and CTPA, but often forget about the value of V/Q scans [8]. Consider this test in:
- Young, otherwise healthy patients with a normal chest x-ray
- CT contrast allergy
V/Q SPECT
V/Q SPECT has been shown to have superior accuracy compared to traditional V/Q and has similar sensitivity, but poorer specificity compared to CTPA for pulmonary embolism [9]. V/Q SPECT eliminates intermediate probability scans, and is reported dichotomously as positive or negative for PE. This avoids the ambiguity of results in traditional V/Q. Robust data is pending regarding its diagnostic utility compared to CTPA.
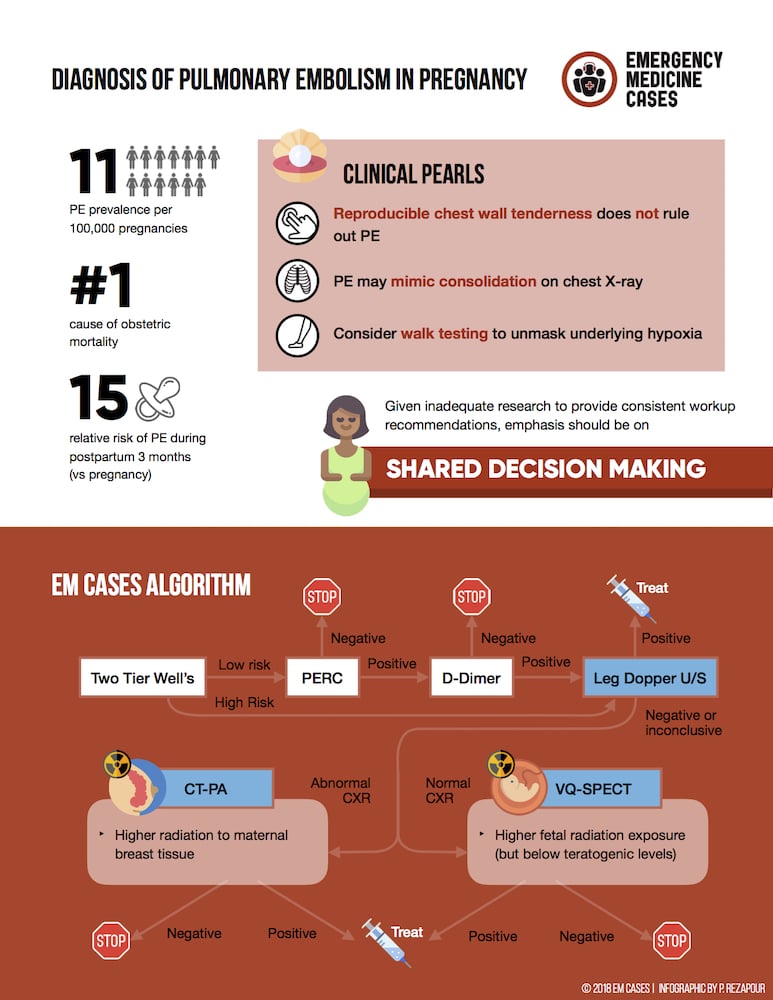
Pregnancy and pulmonary embolism challenges in diagnosis
There are many proposed strategies for working up the pregnant patient for PE, but no diagnostic algorithm has robust enough evidence for strong recommendations [10,11,12]. Pregnant women have generally been excluded from the studies that have provided support for the use of clinical prediction tools and D-dimer in the diagnosis of pulmonary embolism.
Although a trimester-adjusted D-dimer (cutoffs increase by 250 for each trimester) has been suggested for PE in pregnancy, it is not recommended by our experts. While the DiPEP study could not find a D-dimer threshold below which PE could be ruled out in pregnancy [13], there is some observational evidence that a negative D-dimer result rules out PE in otherwise low-risk pregnant patients. A retrospective review of 152 pregnant and post-partum patients who underwent V/Q or CTPA for suspected PE found a sensitivity of 100% but only a specificity of 42% [14]. The American Thoracic Society recommends not using D-dimer in pregnancy [15].
The European Society of Cardiology recommends considering V/Q scan to rule out suspected PE in pregnant women with normal CXR (Class IIB recommendation) and that CTPA should be considered if the CXR is abnormal or if V/Q scan is not readily available (Class IIa recommendation) [16].
Our experts recommend starting with two-tier Wells and PERC, an unadjusted D-dimer if necessary, then moving onto bilateral leg dopplers, and then considering chest imaging based on the CADTH Optimal Strategies for the Diagnosis of Acute Pulmonary Embolism 2018 Recommendations [17].
- Two-tier Wells
- PERC
- D-dimer
- Leg ultrasound
- CTPA or VQ SPECT

From CADTH Optimal Strategies for the Diagnosis of Acute Pulmonary Embolism: Recommendations 2018
Can leg dopplers rule PE in or out? Ultrasound shows a DVT in up to 30-50% of patients with PE, and finding a proximal DVT in patients suspected of having PE is considered sufficient to warrant anticoagulation without further testing [18]. A negative Doppler ultrasound for DVT does not rule out a PE.
Radiation Risk in pregnancy: CTPA vs. V/Q
A CTPA transmits more radiation to the maternal breast tissue, whereas a V/Q scan transmits more radiation to the fetus. There is no hard data here to guide practice and specific strategies remain controversial. However, it is important to realize that both VQ and CTPA fetal radiation dose falls well below teratogenic doses. In the ED, discuss radiation risk with your patient and the radiologist on-call to determine the best imaging modality.
Update 2019: In a prospective study of 510 pregnant women with suspected PE, a pregnancy adapted-YEARS diagnostic algorithm was applied across all trimesters using the following criteria: 1) clinical signs of DVT, hemoptysis, PE most likely diagnosis and D-dimer level (YEARS); and 2) compression ultrasonography for those with symptoms of DVT (if positive, no CTPA was done). CTPA was avoided in 32-65% of patients using this algorithm. Abstract
Update 2021: Pregnancy-Adapted Geneva (PAG) score assessed in a multicenter, prospective study of 395 women with suspected acute pulmonary embolism (PE). PAG score showed high discriminative power to identify low, intermediate, or high pre-test probability of a PE (increasing prevalence of 2.3%, 11.6%, and 61.5% respectively). Abstract
Update 2021: Randomized pulmonary embolism (PE) screening trial of 746 patients admitted for chronic obstructive pulmonary disease (COPD) exacerbation across 18 Spanish hospitals. Active strategy for diagnosis of PE (with D-dimer, and if positive, CT pulmonary angiogram) compared with usual care, did not significantly improve composite outcome of non-fatal new/recurrent venous thromboembolism (VTE), re-admission for COPD, or death at 90 days. Abstract
Departmental pulmonary embolism decision support
Our experts encourage every ED to develop a protocol for PE diagnosis to maintain consistency and promote institutional support for clinicians. If implemented thoughtfully with input from the physician group, this practice could lead to reduced imaging rates and increased diagnostic yield [19]. However, changing ED culture may be challenging, and results depend on the point of implementation to affect diagnostic momentum.
For more on pulmonary embolism on EM Cases:
Ep 113 Pulmonary Embolism Challenges in Diagnosis Part 1
Episode 21: Pulmonary Embolism
Best Case Ever 44 Low Risk Pulmonary Embolism
BCE 77 Pulmonary Embolism Workup in Pregnancy
EM Quick Hits 42 – Subsegmental PE, Trauma Analgesia, Drowning, Polio, Head-up CPR
References
- Writing Group for the Christopher Study Investigators. Effectiveness of Managing Suspected Pulmonary Embolism Using an Algorithm Combining Clinical Probability, D-Dimer Testing, and Computed Tomography. JAMA. 2006;295(2):172-179.
- van der Hulle T, van Es N, den Exter PL, et al. Is a normal computed tomography pulmonary angiography safe to rule out acute pulmonary embolism in patients with a likely clinical probability? A patient-level meta-analysis. Thromb Haemost. 2017;117(8):1622-1629.
- Outcomes following a negative computed tomography pulmonary angiography according to pulmonary embolism prevalence: a meta-analysis of the management outcome studies. J Thromb Haemost. 2018 Jun;16(6):1107-1120.
- Den exter PL, Van es J, Klok FA, et al. Risk profile and clinical outcome of symptomatic subsegmental acute pulmonary embolism. Blood. 2013;122(7):1144-1150.
- Goy J, Lee J, Levine O, Chaudhry S, Crowther M. Sub-segmental pulmonary embolism in three academic teaching hospitals: a review of management and outcomes. J Thromb Haemost. 2015;13(2):214-8.
- Yoo HH, Queluz TH, El dib R. Anticoagulant treatment for subsegmental pulmonary embolism. Cochrane Database Syst Rev. 2014;(4):CD010222.
- ACEP Clinical Policies Subcommittee. Clinical Policy: Critical Issues in the Evaluation and Management of Adult Patients Presenting to the Emergency Department with Suspected Acute Venous Thromboembolic Disease. Ann Emerg Med 2018; 71(5): e59-109.
- Le Roux PY, Pelletier-Galarneau M, De Laroche R, Hofman MS, Zuckier LS, Roach P, et al. Pulmonary scintigraphy for the diagnosis of acute pulmonary embolism: a survey of current practices in Australia, Canada, and France. J Nucl Med. 2016;56(8):1212-7.
- Gutte H, Mortensen J, Jensen CV, et al. Comparison of V/Q SPECT and planar V/Q lung scintigraphy in diagnosing acute pulmonary embolism. Nucl Med Commun. 2010;31(1):82-6.
- Kline JA, Williams GW, Hernandez-nino J. D-dimer concentrations in normal pregnancy: new diagnostic thresholds are needed. Clin Chem. 2005;51(5):825-9.
- Hunt BJ, Parmar K, Horspool K, et al. The DiPEP (Diagnosis of PE in Pregnancy) biomarker study: An observational cohort study augmented with additional cases to determine the diagnostic utility of biomarkers for suspected venous thromboembolism during pregnancy and puerperium. Br J Haematol. 2018;180(5):694-704.
- Leung AN, Bull TM, Jaeschke R, Lockwood CJ, Boiselle PM. Evaluation of Suspected Pulmonary Embolism in Pregnancy. American Journal of Respiratory Critical Care Medicine 2011; 184: 1200-1208.
- Hunt BJ, Parmar K, Horspool K, et al. The DiPEP (Diagnosis of PE in Pregnancy) biomarker study: An observational cohort study augmented with additional cases to determine the diagnostic utility of biomarkers for suspected venous thromboembolism during pregnancy and puerperium. Br J Haematol. 2018;180(5):694-704.
- Choi H, Krishnamoorthy D. The diagnostic utility of D-dimer and other clinical variables in pregnant and post-partum patients with suspected acute pulmonary embolism. Int J Emerg Med. 2018;11(1):10.
- Leung AN, Bull TM, Jaeschke R, et al. American Thoracic Society documents: an official American Thoracic Society/Society of Thoracic Radiology Clinical Practice Guideline–Evaluation of Suspected Pulmonary Embolism in Pregnancy. Radiology. 2012;262(2):635-46.
- Konstantinides S, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033-69, 3069a-3069k.
- Optimal Strategies for the Diagnosis of Acute Pulmonary Embolism: Recommendations. Ottawa: CADTH; 2018 Mar. (CADTH optimal use report; vol.6, no.3c).
- Le Gal G, Righini M, Sanchez O, et al. A positive compression ultrasonography of the lower limb veins is highly predictive of pulmonary embolism on computed tomography in suspected patients. Thromb Haemost. 2006;95(6):963-6.
- Deblois S, Chartrand-lefebvre C, Toporowicz K, Chen Z, Lepanto L. Interventions to Reduce the Overuse of Imaging for Pulmonary Embolism: A Systematic Review. J Hosp Med. 2018;13(1):52-61.
- Additional Podcast References
- Van Mens TE, Scheres LJ, De jong PG, Leeflang MM, Nijkeuter M, Middeldorp S. Imaging for the exclusion of pulmonary embolism in pregnancy. Cochrane Database Syst Rev. 2017;1:CD011053.
- Van der, Mairuhu A, Tromeur C, Couturaud F, Huisman M, Klok F. Use of clinical prediction rules and D-dimer tests in the diagnostic management of pregnant patients with suspected acute pulmonary embolism. Blood Rev. 2017;31(2):31-36.
- Lin MP, Probst MA, Puskarich MA, et al. Improving perceptions of empathy in patients undergoing low-yield computerized tomographic imaging in the emergency department. Patient Educ Couns. 2018;101(4):717-722.
- Kline JA, Neumann D, Raad S, et al. Impact of Patient Affect on Physician Estimate of Probability of Serious Illness and Test Ordering. Acad Med. 2017;92(11):1607-1616.
Other FOAMed Resources on PE imaging, subsegmental, and PE in pregnancy
EP Monthly on CTPA – Is this test just a little too good?
EM Literature of Note on Again with the failings of CTPA
Rebel EM on The DiPEP study
EM Docs on Controversies in imaging and treatment of subsegmental PE
Rebel EM on ACEP Clinical Policy on Acute VTE 2018
Drs. Helman and Lang have no conflicts of interest to declare. Dr. DeWit conducts research funded by Bayer.
Now test your knowledge with a quiz.




Leave A Comment