This is part 2 of our podcast series on liver disease for the ED doc with Walter Himmel and Brian Steinhart. We clear up the confusing balance between thrombosis and bleeding in liver patients, the elusive diagnosis of portal vein thrombosis, spontaneous bacterial peritonitis diagnosis and treatment and some tips and tricks on paracentesis. We answer questions such as: How is it that liver patients are at increased risk for both thrombosis and bleeding? How should we interpret the INR of liver patients in the setting of thrombosis or bleeding? Should a liver patient with an elevated INR be placed on an anticoagulant for portal vein thrombosis or pulmonary embolism? Is there a role for TXA in the bleeding liver patient? Why were PCCs rarely indicated in the liver patient in the past, and now being reconsidered? Do you need to obtain an INR before performing a paracentesis? At what platelet count is it safe to perform a paracentesis? What are the indications for giving albumin after paracentesis? for spontaneous bacterial peritonitis? and many more…
Podcast production, sound design & editing by Anton Helman. Voice editing by Raymond Cho.
Written Summary and blog post by Anton Helman & Jennifer He, November, 2020
Cite this podcast as: Helman, A. Himmel, W, Steinhart, B. Episode 149 Liver Emergencies: Thrombosis and Bleeding, Portal Vein Thrombosis, SBP, Paracentesis Tips and Tricks. Emergency Medicine Cases. November, 2020. https://emergencymedicinecases.com/liver-emergencies-thrombosis-bleeding-portal-vein-thrombosis-sbp-paracentesis. Accessed [date]
For part 1 of this series on Liver Emergencies go to Episode 148 Liver Emergencies: Acute Liver Failure, Hepatic Encephalopathy, Hepatorenal Syndrome, Liver Test Interpretation & Drugs to Avoid
Liver patients are at higher risk of clotting than of bleeding
There is a complex imbalance of coagulation factors in the liver patient that is essential to understand for effective management of thrombotic and hemorrhagic events. In general, patients with liver disease are more likely to develop thrombotic disease than they are bleeding diathesis, even if the INR is elevated.
Increased risk of thrombosis: patients with cirrhosis may have decreased protein S and C more so than a reduction in Factors 2, 7, 9, 10 and this would favor thrombosis, rather than bleeding.
Pitfall: a common pitfall is assuming that a liver patient with an elevated INR is protected from thrombotic events. An elevated INR level does not imply that the patient is protected from pulmonary embolism or portal vein thrombosis.
Hypoalbuminemia can be considered a risk factor for thrombosis risk in liver patients
A potential predictor of venous thromboembolism in a cirrhotic patient (assumed to be “auto-anticoagulated” based on an elevated INR value) is serum albumin. It is hypothesized that lower serum albumin concentration is a surrogate for decreased protein synthesis by the liver and thus decreased production of endogenous anticoagulant factors such as Protein C and S. In short, if the albumin is low, the patient may be at increased risk of thromboembolic events.
Treatment of thrombotic liver disease: cirrhotic vs non-cirrhotic
Treatment of thrombotic liver disease varies with the presence or absence of cirrhosis. Cirrhosis increases the risk of catastrophic bleeding from esophageal or gastric varices if the patient is anticoagulated. Patients with cirrhosis who require anticoagulation need careful evaluation with endoscopy for varices and these varices are best definitively treated with banding. Consideration should also be given to clot removal, transjugular intrahepatic portosystemic shunt procedure, or surgical shunting as an alternative to anticoagulation.
Portal vein thrombosis (PVT)
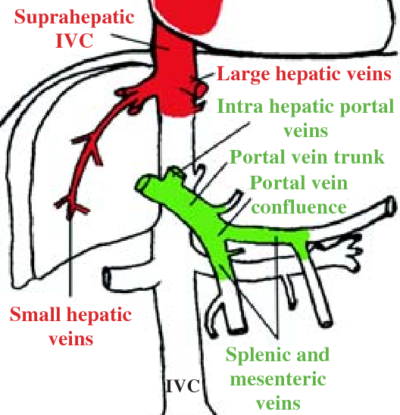
The portal vein is formed from the confluence of splenic and superior mesenteric veins that drain the spleen and small intestines. The occlusion of the portal vein by a thrombus occurs in cirrhotic patients and other patients in a prothrombotic state, such as those with active cancer. Liver disease is a risk factor for venous thromboembolism regardless of the INR level.
Most PVTs are picked up coincidentally, as 20-40% of patients with PVT are asymptomatic, 20-50% present with abdominal pain, and 25-40% present with GI bleed. The American Association for the Study of Liver Disease (AASLD) suggests that we consider acute PVT in “any patient with abdominal pain of more than 24 hours duration,” independent of the presence of fever or ileus.
Physical examination in patients with PVT is highly variable and depends on onset and extension of occlusion. Similarly, laboratory analysis shows a spectrum of non-patterned and non-specific values of CBC and metabolic panel with typically normal transaminase levels, unless an underlying condition such as cirrhotic liver failure or hepatitis is present. Liver function is usually preserved in PVT.
Have a low threshold to obtain imaging for liver patients with abdominal pain or GI bleed to look for PVT. CT with contrast is the imaging modality of choice. Doppler ultrasound is an excellent alternative to CT with a sensitivity of 89% and specificity of 92% for portal vein thrombosis.
Management of portal vein thrombosis (PVT) in patients with chronic liver disease
Step 1: evaluate for bleeding risk (eg: gastroesophageal varices)
Step 2: consider prophylactic treatment for variceal bleeding before anticoagulation; if varices are present, initiate treatment with beta blockers and/or arrange for rubber band ligation in patients with large varices or in those with previous variceal bleeding
Step 3: ensure platelets > 50,000 and fibrinogen > 100 mg/dL; may consider vitamin K for bleeding however it often does not result in an improved INR in liver patients
Step 4: start spontaneous bacterial peritonitis prophylaxis – ceftriaxone 1 g IV daily until patient is taking food orally
Step 5: start anticoagulation, preferably in consultation with a hematologist
LMWH: consider using LMWH (as VKA monitoring may be difficult due to variations in INR with cirrhosis and unfractionated heparin is generally not recommended as the baseline PTT is often prolonged) and use fixed or weight-adjusted doses.
Bleeding in the liver patient
Key concept: most life-threatening GI bleeds in liver patients are caused primarily by portal hypertension and only secondarily to altered hemostasis.
Altered hemostasis is multifactorial including decreased platelet count and function, increased fibrinolysis and Vitamin K deficiency. Liver disease impairs factor synthesis, which leads to decreased Vitamin K dependent factors (II, VII, IX, X).
Pitfall: relying on INR, PT, PTT in a cirrhotic patient who is not taking Warfarin to risk stratify for bleeding can be misleading. The INR measures Vitamin K dependent factors, does not reflect the entire coagulation cascade, and its elevation is not necessarily indicative of the need for fresh frozen plasma (FFP) or prothrombin complex concentrates (PCCs), as it does not contribute significantly to development of variceal bleeding or other sources of hemorrhage in these patients.
ED management of bleeding in the liver patient
Key concept: do not over-transfuse. Carefully weigh the benefits of giving additional blood products in an attempt to optimize coagulopathy against the potential for increasing portal pressure (portal hypertension), especially in a patient with varices, even if this is not the site of acute bleeding.
1.Obtain source control – obtain source control of the bleed; when accomplished rapidly, it may obviate the need for administration of blood products
2.Massive transfusion protocol – consider activation of a massive transfusion as per your hospital protocol if the patient is hemorrhaging rapidly. A close look at the TRIGGER Study out of the UK, reveals that only 5% of variceal bleeds require a massive transfusion protocol. In other words, 95% of GI bleed patients require only red cell transfusions. Over-activation of massive transfusion protocols lead to unnecessary ![]() complications such as Transfusion Associated Circulatory Overload (TACO) and wasted blood products.
complications such as Transfusion Associated Circulatory Overload (TACO) and wasted blood products.

Dr. Jeannie Callum’s expert opinion indications for MTP in UGIB
Andrew Petrosoniak on MTP from EMU
3. Employ TEG or ROTEM to guide management of the bleeding liver patient; if unavailable evaluate platelet count and fibrinogen level. Do not rely on the INR!
- Pool of platelets to maintain a platelet count greater than 50,000 for active, severe, or CNS bleeding. If there is severe ongoing bleeding and platelet function is thought to be impaired, the clinician may consider platelet transfusion independent of platelet count.
- Cryoprecipitate or fibrinogen to maintain a fibrinogen level > 100 mg/dL.
4. Consider Vitamin K – Administer 10 mg of Vitamin K to those at risk of Vitamin K deficiency. Based on expert opinion, it is reasonable to administer Vitamin K in bleeding or non-bleeding liver pts if the INR is greater than 5. However, if the INR is elevated for a reason other than Vitamin K deficiency, then Vitamin K is unlikely to be helpful, and may promote thromboembolic events.
TXA in liver patients with life-threatening bleeding
TXA is unlikely to benefit and may cause harm in the liver patient with internal bleeding. The HALT-IT trial, a large, multicenter randomized controlled trial of TXA vs placebo for acute GI bleed showed no benefit of giving TXA on 5d mortality and a small increase in venous thromboembolic events and seizures. Since liver patients are at increased risk for thromboembolism, the harms are likely greater than seen in this study.
TXA may be considered as local/topical therapy for epistaxis or oral bleed.
PCCs, FFPs, and rFVIIa in liver patients with life-threatening bleeding
PCCs, FFPs, and rFVIIa should generally be avoided in liver patients as they are unlikely to be effective and carry a thrombotic risk. There is recent expert opinion suggesting that PCCs may be indicated in a minority of patients. If you are considering giving these medications in the liver patient with life-threatening bleeding it is advisable to discuss the decision with a hematologist first.
Bacterial Peritonitis
Do not rule out spontaneous bacterial peritonitis (SBP) with a normal abdominal exam
SBP presents with ascites, fever, abdominal pain, altered mental status and/or hypotension. Other clues include new renal failure, hypothermia and unexplained leukocytosis.
As per the AASLD, a diagnostic paracentesis should be considered in all patients with new-onset ascites. Paracentesis provides the definitive diagnosis for bacterial peritonitis with PMNs ≥ 250 cells/mm3 in ascitic fluid.
Pitfall: do not rule out SBP based on a “benign” abdomen; ascites can prevent the development of classic peritoneal signs by creating a separation between the visceral and parietal peritoneum.
Recognize that liver patients are at risk for sepsis and dying from sepsis from any source. Do not attribute elevated lactate to the liver disease itself. Assume sepsis until proven otherwise. Have a low threshold to order imaging to rule out a secondary cause of the bacterial peritonitis as the abdominal exam in SBP can be deceptively benign.
It is important to distinguish between spontaneous bacterial peritonitis and secondary bacterial peritonitis
The mortality rate of secondary bacterial peritonitis has been reported to be as high as 66% without appropriate surgical intervention. It may be associated with processes such as perforation peritonitis and intra-abdominal abscess.
Suspect secondary bacterial peritonitis when 2 or more of the following ascitic fluid findings are present:
- Total protein greater than 1 g/dL.
- Glucose less than 50 mg/dL.
- Lactate dehydrogenase greater than upper limit for serum.
Pearl: have a low threshold for abdominal imaging in liver patients with fever of unknown source or abdominal pain to rule out a secondary cause of bacterial peritonitis.
Spontaneous Bacterial Peritonitis (SBP) management in the ED
- Administer antibiotics early – cefotaxime 2 g IV q8h or ceftriaxone 2 g IV
- The AASLD recommends IV albumin (1.5 g/kg within 6 hours of diagnosis of SBP followed by 1.0 g/kg on day 3) in patients with a serum creatinine >1 mg/dL or BUN >30 mg/dL or bilirubin >4 mg/dL; it is also reasonable to consider albumin infusion in patients undergoing large volume paracentesis (>5 liters)
- Consider pressor support as needed, octreotide if renal failure present
- Transjugular intrahepatic portosystemic shunt (TIPSS) should be considered in patients with refractory ascites
Paracentesis considerations
Is there an INR above which (or a platelet count below which) a paracentesis should be withheld?
Prospective literature supports no increased risk of bleeding after the performance of a paracentesis in the setting of an INR < 8.0 or platelet count > 20,000/ml. Minor abnormalities in INR or platelet count are not a reason to defer the performance of a paracentesis when clinically indicated.
Consider albumin in select population following large-volume paracentesis for ascites
The evidence for administering albumin for patients undergoing paracentesis for spontaneous bacterial peritonitis remains somewhat controversial. Our experts suggest considering giving albumin if removing more than 5 L of ascites in the context of possible SBP. The evidence for benefit of albumin is less convincing in the context of large volume paracentesis in the absence of SBP.
As per the AASLD
Paracentesis of > 5 L: albumin (as 20% or 25% solution) should be infused at a dose of 8 g albumin/L of ascites removed. (Quality of evidence: high; Recommendation: strong).
Paracentesis of < 5 L: albumin (as 20% or 25% solution) can be considered at a dose of 8 g albumin/L of ascites removed in patients with acute-on-chronic liver failure or in those at high risk of post-paracentesis acute kidney injury. (Quality of evidence: low; Recommendation: weak).
Role of spironolactone in patients with ascites
First presentation of moderate ascites: spironolactone monotherapy (starting dose 100 mg, increased to 400 mg) can be considered.
Recurrent severe ascites: combination therapy with spironolactone (starting dose 100 mg, increased to 400 mg) and furosemide (starting dose 40 mg, increased to 160 mg) if faster diuresis needed.
Paracentesis technique tips and tricks
A variety of techniques have been described for paracentesis. The best videos we found are here and here . To summarize our experts’ tips and tricks:
- Make use of POCUS to assist with paracentesis
- Choose a site of puncture lateral to the rectus sheath
- Consider using a pigtail catheter
- To minimize fluid leakage consider the Z-track technique (reviewed here). This involves puncturing the skin at the desired site, then pulling the skin either caudally, downwards or cephalad before advancing the needle and catheter into the peritoneum. Alternatively, the skin can be pulled downward first, followed by needle puncture through skin and into the peritoneal cavity. This provides two separate entry points, reducing the likelihood of ascites leaking. If a leak occurs, the patient can be place in the lateral decubitus position (puncture side up) to limit leak, and once the area is adequately dry (with gauze), a few layers of 2-octyl-cyanoacrylate tissue glue adhesive can be applied, essentially closing the site.
Key Take Home Points for Liver Emergencies: Thrombosis and Bleeding, Portal Vein Thrombosis, Spontaneous Bacterial Peritonitis and Paracentesis
- Do not assume that the liver patient with a high INR is anticoagulated; they may still be at risk for thrombosis
- Keep your differential wide in liver patients with abdominal pain, have a low threshold for abdominal imaging, and think specifically about the possibility of portal vein thrombosis and bacterial peritonitis even in the patient with a “benign” abdomen on physical exam
- IV albumin should be considered in the patient with acute liver failure and/or bacterial peritonitis and/or paracentesis >5L of ascitic fluid
- Do not forget to order a fibrinogen level in the liver patient with life-threatening bleeding and give cryoprecipitate or fibrinogen to keep the fibrinogen level > 100
- It is considered generally safe to perform a paracentesis in a liver patient with an INR as high as 8 and a platelet count as low as 20,000
- Consider use of POCUS, a pigtail catheter, Z-track technique and tissue glue adhesive for paracentesis
References
- Harrison MF. The Misunderstood Coagulopathy of Liver Disease: A Review for the Acute Setting. West J Emerg Med. 2018;19(5):863-871.
- EASL Clinical Practice Guidelines: Vascular diseases of the liver. J Hepatol. 2016;64(1):179-202.
- O’Mara, Susan R., and Kulleni Gebreyes. “Hepatic disorders.” A comprehensive Study Guide: Tintinalli’s Emergency Medicine. 8th ed. Mc Graw Hill 525 (2016).
- Mancuso A. Management of portal vein thrombosis in cirrhosis: More shadows than lights. Dig Liver Dis. 2017;49(2):228.
- Tessler, F. N., Gehring, B. J., Gomes, A. S., Perrella, R. R., Ragavendra, N., Busuttil, R. W., & Grant, E. G. (1991). Diagnosis of Portal vein thrombosis: Value of color Doppler imaging. American Journal of Roentgenology, 157(2), 293-296.
- Callcut, R. A., Cotton, B. A., Muskat, P., Fox, E. E., Wade, C. E., Holcomb, J. B., Schreiber, M. A., Rahbar, M. H., Cohen, M. J., Knudson, M. M., Brasel, K. J., Bulger, E. M., Del Junco, D. J., Myers, J. G., Alarcon, L. H., & Robinson, B. R. (2013). Defining when to initiate massive transfusion. Journal of Trauma and Acute Care Surgery, 74(1), 59-68.
- Runyon BA; AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009 Jun;49(6):2087-107. doi: 10.1002/hep.22853. PMID: 19475696.
- Soriano, G., Castellote, J., Álvarez, C., Girbau, A., Gordillo, J., Baliellas, C., Casas, M., Pons, C., Román, E. M., Maisterra, S., Xiol, X., & Guarner, C. (2010). Secondary bacterial peritonitis in cirrhosis: A retrospective study of clinical and analytical characteristics, diagnosis and management. Journal of Hepatology, 52(1), 39-44.
- Bernardi M, Caraceni P, Navickis RJ, Wilkes MM. Albumin infusion in patients undergoing large‐volume paracentesis: a meta‐analysis of randomized trials. Hepatology. 2012 Apr;55(4):1172-.
- Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999 Aug 5;341(6):403-
- Salerno F, Navickis RJ, Wilkes MM. Albumin infusion improves outcomes of patients with spontaneous bacterial peritonitis: a meta‐analysis of randomized trials. Clin Gastroenterol Hepatol. 2013 Feb;11(2):123.
- Kwok CS, Krupa L, Mahtani A, et al. Albumin reduces paracentesis-induced circulatory dysfunction and reduces death and renal impairment among patients with cirrhosis and infection: a systematic review and meta‐ Biomed Res Int. 2013;2013:295153.
- Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, Castells L, Vargas V, Soriano G, Guevara M, Ginès P, Rodés J. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999 Aug 5;341(6):403-9. doi: 10.1056/NEJM199908053410603. PMID: 10432325.
- Hale, B. R., & Girzadas, D. V. (2001). Application of 2-octyl-cyanoacrylate controls persistent ascites fluid leak. The Journal of Emergency Medicine, 20(1), 85-86.
Other FOAMed on Liver Emergencies
CanadiEM Crack Cast Liver and Biliary Tract
PulmCrit Coagulopathy management in the bleeding cirrhotic
Washington U Journal Club on Albumin for Patients with Paracentesis
Drs. Helman, Himmel and Steinhart have no conflicts of interest to declare
Now test your knowledge with a quiz.





These two podcasts on liver emergencies have been absolutely amazing and the pearl on INR in liver patients,practice changing! Your podcasts have been extremely educative and informative and brings the knowledge of these wonderful experts to our doorsteps.Thank you for all your hard work,it certainly is not in vain!
Natalie-EM registrar (Zambia/South Africa)