There is perhaps no single diagnosis where we see such a huge practice variation than in the management of spontaneous pneumothorax. Even the definition of what a large, tube-in-the-chest-worthy pneumothorax is different depending on where you practice. Management options for small and large spontaneous pneumothorax are all over the place – observation alone, pigtail catheter with Heimlich valve, needle aspiration, large-bore chest tube, underwater seal or suction. There are significant complications associated with each of these options. What’s the best approach to the management of spontaneous pneumothorax? Dr. Gil Yehudaiff, EM physician from North York General in Toronto and Dr. Mehdi Tahiri, thoracic surgeon from McGill University in Montreal and Anton answer questions such as: what is the role of PoCUS in the diagnosis and management of spontaneous pneumothorax? Which large pneumothoraces can be managed without needle aspiration or a chest tube? What are the most common reasons for small-bore pigtail catheter failure? When can a chest tube be safely removed and how should it be removed safely? How often do CXRs need to be repeated in patients with spontaneous pneumothorax? and many more…
Podcast production, sound design & editing by Anton Helman; voice editing by Sheza Qayyam
Written Summary and blog post by Humna Amjad & Gil Yehudaiff; edited by Anton Helman July, 2021
Cite this podcast as: Helman, A. Tahiri, M. Yehudaiff, G. Management of Spontaneous Pneumothorax. Emergency Medicine Cases. July, 2021. https://emergencymedicinecases.com/management-spontaneous-pneumothorax. Accessed [date]
The content of this summary and podcast refers only to the patient with a primary spontaneous pneumothorax under the age of 50. The following does not apply to the patient with a coexisting hemothorax, secondary pneumothorax (eg. COPD, lung cancer), hydrothorax, iatrogenic pneumothorax, traumatic pneumothorax or tension pneumothorax.
What is the role of PoCUS in the diagnosis and management of spontaneous pneumothorax?
PoCUS has excellent test characteristics for the diagnosis of pneumothorax with a sensitivity of 90.9% and specificity of 98.2% based on pooled data. While upright CXR is also very accurate for the diagnosis of clinically significant pneumothorax, supine CXR has been found to have a sensitivity of only 50.2%. Since most trauma patients remain in a supine position during their ED evaluation, POCUS is most useful as part of E-FAST when a dichotomous answer as to whether or not the patient has a pneumothorax is required urgently. In the setting of spontaneous non-traumatic pneumothorax, PoCUS has a limited role as there is not enough evidence to suggest that PoCUS can be used to accurately quantify the size of the pneumothorax, and current guidelines are based primarily on CXR size measurements of the pneumothorax. Attempts have been made to quantify pneumothorax volume using PoCUS. A study in 2014 in Italy compared lung ultrasound to CXR and CT for quantifying pneumothorax volume. They found that using a Lung Point at the mid-axillary line could help differentiate between small or large pneumothorax based on guideline CXR definitions. However, the accuracy was best for PoCUS for small pneumothoraces only.
Does size matter when it comes to spontaneous pneumothorax management?
Defining the size of a pneumothorax guides subsequent management decisions. Unfortunately there is no agreed upon universal definition of large pneumothorax, with several accepted definitions:
- The American College of Chest Physicians – apex-cupola distance >3 cm
- The British Thoracic Society – interpleural distance at the hilum >2 cm
- Belgian guidelines – dehiscence over the entire length of the lateral chest wall
- Light’s index (see image below)- calculative approach that involves the diameter of the collapsed lung (L) and the diameter of the inner hemithorax at the hilum (H) with estimated pneumothorax size = (1 – L3/H3) x 100 of ≥20% defined as large
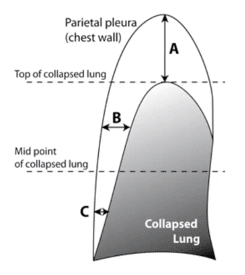
- Collin’s Method (see image below)- A+B+C > 6cm
Management guidelines for spontaneous pneumothorax vary widely and are based mainly on expert opinion
Guidelines exclude patients that are physiologically unstable with any of the following abnormal vital signs or those with a secondary pneumothorax, as these patients generally require a chest-tube and admission:
- SBP < 90 mmHg
- HR > SBP
- RR > 30 breaths/min
- SpO2 < 90%
A) Small primary spontaneous pneumothorax, mildly symptomatic
- Patients with small primary spontaneous pneumothorax who have stable vital sign and are mildly symptomatic can be observed in the ED with a repeat CXR at 4 hours
- If the pneumothorax appears stable, they can be safely discharged home with a repeat x-ray in 24-72 hours
- It is reasonable to provide supplemental oxygen while waiting for repeat CXR as there is retrospective data suggesting that it may hasten lung expansion
B) Large spontaneous primary pneumothorax
Large spontaneous primary pneumothorax
Guidelines vary:
- Chest 2001 – completely expert opinion-based, suggests small bore “pigtail” catheter for all large pneumothoraces
- British Thoracic Society 2010 – conservative management with large pneumothorax may be appropriate and needle aspiration is a first option; small-bore chest tube is next option
- Belgian Respiratory Society 2005 – if large, start with needle aspiration or small-bore chest tube
- European Respiratory Society 2015 – focus on patient symptoms; observation alone for large stable pneumothorax
Conservative management of stable mildly symptomatic large primary spontaneous pneumothorax: A paradigm shift
For patients age < 50 years with large primary spontaneous pneumothorax who are mildly symptomatic a conservative approach can be considered whereby the patient is monitored for 4 hours in the ED and CXR is repeated. If the CXR shows an increase in the size of the pneumothorax, further intervention would be required (ie: needle aspiration or small-bore chest tube). If the pneumothorax remains stable, vitals are normal, pain is controlled, and the patient is ambulating comfortably, these patients can be safely discharged home with repeat CXR within 24 hours without a chest tube.
The evidence for this conservative approach versus chest tube insertion comes from the first RCT looking at this question – a 2020 multicenter, prospective, randomized, noninferiority trial in Australia and New Zealand. They randomized 316 patients, aged 14-50, with first presentation, unilateral, spontaneous pneumothorax, deemed to be moderate to large in size using Collins method (see above).
They found that there was less lung re-expansion at 8 weeks, decreased pneumothorax recurrence, fewer serious adverse events and a trend toward more favourable patient satisfaction with the conservative approach.
- Lung re-expansion at 8 weeks with conservative approach 94.4% vs chest tube 98.5% (conservative approach was non-inferior)
- Pneumothorax recurrence at 12 months with conservative approach 8.8% vs chest-tube 16.8% (ARR = 8%; NNH = 12.5)
- Mean of 4.5 fewer days off work with conservative approach
- Fewer adverse and serious adverse events with conservative approach
- Trend towards more favorable patient satisfaction with conservative approach
- 15% in conservative arm required an intervention
In patients with large pneumothoraces who have debilitating symptoms, a conservative approach should not be considered, and the management involves either a small-bore chest tube with Heimlich valve or a needle aspiration followed by small-bore chest tube if needle aspiration fails. Patients with a complete or near-complete collapse of a lung should not be considered for a conservative approach according to our experts.
In well-selected patients, a conservative approach is non-inferior to an intervention-based approach for management of large stable spontaneous pneumothorax. Chest tubes appear to lead to more adverse events, more pain and more recurrence.
Should needle aspiration of spontaneous pneumothorax be the first line intervention?
Evidence from two RCTs that enrolled patients with primary large spontaneous pneumothorax requiring decompression found that while needle aspiration had a lower rate of immediate success compared to chest tube insertion (50-68% vs 80.6%), time to complete resolution were equivalent, needle aspiration showed a trend toward reduced recurrence of pneumothorax at 12 months (4% recurrence with aspiration vs 12.9% with chest tube) and was associated with fewer serious adverse events. Shared decision making with patients is apt in these situations. If the patient is stable and prefers to avoid chest tube insertion, needle aspiration can be considered as a first line option.
Procedure for Needle Aspiration of pneumothorax
Different catheters can be used to perform needle aspiration – be familiar with the devices available at your institution.
NEJM Procedural video for needle aspiration of pneumothorax
- Position patient in semi-upright position to allow air to collect at the apex.
- Preferred location is the 2nd intercostal space at midclavicular line.
- Properly drape patient and adorn sterile gown and gloves.
- Using 25-gauge needle, inject a wheel of lidocaine at the superior edge of 3rd rib, at midclavicular line.
- Switch to 22-gauge and anesthetize deeper tissues. Insert needle perpendicular to the skin, always aspirating first.
- Needle should be positioned over 3rd rib to avoid injuries to intercostal vessels and nerves.
- When pleural space is penetrated, air bubbles will appear with aspiration of the needle. Take notice of this depth to be used a reference point for the next step.
- Connect the over-the-needle catheter to the 10-ml lidocaine syringe. Use the same landmarks and advance the needle, this time by a few more millimeters to allow the catheter tip to fully penetrate the pleural space.
- Remove the catheter needle and the 10-ml syringe as the patient exhales or coughs.
- Obstruct the opening of the catheter quickly to prevent air entry into the pleural space.
- Attach tubing with the three-way stopcock to the catheter and use the 50-ml syringe to aspirate air.
- When manipulating the stopcock, ensure the pleural space is never open to the environment.
- Continue manual aspiration until you cannot aspirate any more air. Remove catheter and put a sterile dressing on the insertion site.
- Repeat CXR should be taken with the patient in an upright position.
Procedure for placing small-bore chest tube “pigtail” catheter using Seldinger-style technique
All patients with primary spontaneous pneumothorax <50 years of age who require decompression should have a small-bore 14F pigtail catheter with Heimlich valve rather than a large bore chest tube that has traditionally been used in the trauma setting.
Our experts’ tips and tricks
- The best location to place the pigtail catheter is that which minimizes the skin to pleura distance, which depends on patient body habitus; if in doubt aim higher to avoid puncturing the diaphragm or liver
To manage pain associated with a chest tube, consider flushing the tube with 10-20 ml of lidocaine or bupivacaine; flush with NS afterwards, and do not immediately reconnect to suction so as to avoid suctioning out the anesthetic
- Serratus anterior plane nerve block is another option for pain control
- Secure the catheter well with 1-0 silk sutures and ample dressing to minimize dislodgement
- Check for an air leak by simply submerging the Heimlich valve in a cup of water. Ask the patient to cough 2-3 times forcefully; if there are no bubbles formed in the water after the first cough (which may be related to residual air in the chest tube), then an air leak is unlikely
- For large pneumothorax, especially if collapsed for days, get the patient to take a few big coughs to help re-expand the lung
- Have the patient hum while removing the chest tube to aid in expansion of the lung
Pigtail catheter procedural video for spontaneous pneumothorax from EM Cases Summit
Management after small-bore chest tube placement for spontaneous pneumothorax
- Check for air leak – if present, discharge home with pigtail in place with the valve open and repeat CXR q24h until there is resolution of air leak.
- If there is no air leak, close the valve, repeat CXR in 4 hrs and if the lung still expanded you can remove the pigtail catheter; however, if the lung collapses again, open the valve again and the pigtail catheter needs to stay in.
Removal of small-bore pigtail catheter
Patients must first have a CXR demonstrated near complete or complete lung re-expansion before considering removal of the chest tube. The removal should be done carefully, in a stepwise process to decrease the risk of air leaks into the pleural space.
-
- Check for an air leak by either connecting the chest tube to an underwater seal device, or by submerging the Heimlich valve in a cup of water. Ask the patient to cough 2-3 times forcefully. If there are no bubbles formed in the water after the first cough (which may be related to residual air in the chest tube), then an air leak is unlikely and you may proceed to step 2.
- Clamp the chest tube/close the valve using the 3-way stopcock. Repeat the CXR in 4 hours. If the pneumothorax has not enlarged, then the chest tube is unlikely providing any benefit and may be removed.
- Consider repeating a CXR post chest tube removal to ensure expansion of the lung.
Management after small-bore chest tube placement when decompression fails
If there is no/minimal improvement after chest tube placement, apply suction (apply chest tube to underwater seal + suction at -20cmH2O) and repeat the CXR in 1 hr. If there is improvement with suction, they should be admitted with ongoing suction.
If there is minimal improvement, admit to hospital with a large-bore chest tube after ruling out any problems with the small-bore chest tube such as a kinked tube.

Example algorithm for management of spontaneous pneumothorax adapted from North York General Hospital for educational purposes only
pdf version of example algorithm for management of spontaneous pneumothorax
5 Common reasons for small-bore chest tube pigtail catheter failure
- Large air leak (bronchopleural fistula)
- Fluid blocking the catheter/tubing
- Kinking or dislodgement of the catheter
- Failure to ensure stopcock is in the proper position when connected to Heimlich valve or underwater seal +/- suction
- Dysfunctional Heimlich valve
Who can be safely sent home? Indications for discharge of patients with primary spontaneous pneumothorax
All patients with a known secondary cause for their pneumothorax and patients >50 years old with a first time spontaneous pneumothorax should be presumed to have a secondary cause and should be admitted to hospital.
A study from The Lancet in 2020 of 236 patients with stable large primary spontaneous pneumothorax randomized to pigtail + Heimlich valve and ambulatory care vs usual British care (aspiration +/- chest tube and admission) found that 15% who were discharged from the ED required readmission vs 19% who were admitted required readmission. There were more minor adverse events in ambulatory group. Based on this study, ambulatory care is reasonable for those who do not require a small-bore chest tube + suction, but enforces the importance of timely follow-up (especially for those with a pigtail + Heimlich valve in situ).
How often are follow-up CXRs required in the management of spontaneous pneumothorax?
- For patients without a chest tube obtain another CXR at 24-72 hrs post ED discharge, and then again at 1 week, 2 weeks, 4 weeks, 8 weeks, or until complete resolution.
- For patients with a chest tube obtain a daily CXR until resolution of air leak and removal of chest tube. Only a single view PA CXR is required.
Patients with small primary spontaneous pneumothorax or large mildly symptomatic pneumothorax that understand the follow up instructions can be discharged home. All patients being discharged home should be given instructions for follow up and care.
Discharge and follow-up of patients with spontaneous pneumothorax
While there is regional variation in follow-up it is reasonable for patients with small pneumothoraces, or those with large ones that have become small via needle aspiration or chest tube (that has been removed) to follow up with their primary care provider. All other patients should be followed up by thoracic or general surgery. Patients with ≥2 occurrences of a spontaneous pneumothorax on the same side should be referred to thoracic surgery for consideration for surgery.
Smoking cessation
The recurrence rate of primary spontaneous pneumothorax is 30%. Smoking is a major risk factor for spontaneous pneumothorax. Smoking cessation has been shown to decrease recurrence rates and studies suggest that the ED, during management of a smoking-related disease, is the ideal time and place to counsel patients about smoking cessation and provide follow up for smoking cessation services.
Discharge instructions should include asking the patient to take deep breaths and cough 10 times each hour awake. This is thought to decrease the risk for pneumonia. This can be done by holding a pillow tightly against the incision when coughing. Instruct the patient not to sleep on the side of the catheter so as to minimize the chance of dislodgement.
Example of patient written discharge instruction handout for educational purposes only
Take home points for management of primary spontaneous pneumothorax
- Management decisions are CXR-based, thus every patient needs a CXR even though PoCUS is accurate and has an important role in trauma and critically ill patients
- Choose a definition of large pneumothorax from the American, British or Belgian guidelines and stick with it
- Some patients with large spontaneous pneumothoraces (age < 50 years old, normal vitals, mildly symptomatic, not near/total collapse of the lung) do not need a chest tube or needle aspiration. In the ED, they can receive supplemental oxygen, and a repeat CXR in 4 hours; if stable, they can be discharged with repeat CXR in 24hrs
- Consider needle aspiration first, as an alternative to a chest tube in patients with large symptomatic pneumothoraces who prefer to avoid chest tubes
- Do not forget to check for air leak with the simple “dunking of the Heimlich valve in a cup of water” trick, and getting the patient to cough, before pulling the tube
- Know when to use suction, who can be safely discharged home and who needs to see a thoracic surgeon
- Consider getting together with your ED group and coming up with your own management algorithm that suits your practice environment; it may prevent a lot of confusion, be better for patient care, and improve resource utilization.
Other EM Cases resources on pneumothorax management:
Journal Jam 2: Small bore chest tube and outpatient management of pneumothorax
Episode 31: LP, spontaneous pneumothorax and ultrasound guided fracture reduction
References
- Alrajhi K, Woo MY, Vaillancourt C. Test characteristics of ultrasonography for the detection of pneumothorax: a systematic review and meta-analysis. Chest. 2012 Mar;141(3):703-708.
- Volpicelli, G., Boero, E., Sverzellati, N., Cardinale, L., Busso, M., Boccuzzi, F. Frascisco, M. F. (2014). Semi-quantification of pneumothorax volume by lung ultrasound. Intensive Care Medicine, 40(10), 1460-1467.
- Baumann, M. H., Strange, C., Heffner, J. E., Light, R., Kirby, T. J., Klein, J., Luketich, J. D.,Panacek, E. A., Sahn, S. A., & AACP Pneumothorax Consensus Group (2001). Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest, 119(2), 590–602.
- MacDuff, A., Arnold, A., Harvey, J., & BTS Pleural Disease Guideline Group (2010). Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax, 65 Suppl 2, ii18–ii31.
- De Leyn, P., Lismonde, M., Ninane, V., Noppen, M., Slabbynck, H., Van Meerhaeghe, A., Van Schil, P., & Vermassen, F. (2005). Guidelines Belgian Society of Pneumology. Guidelines on the management of spontaneous pneumothorax. Acta chirurgica Belgica, 105(3), 265–267.
- Tschopp, J. M., Bintcliffe, O., Astoul, P., Canalis, E., Driesen, P., Janssen, J., Krasnik, M.,Maskell, N., Van Schil, P., Tonia, T., Waller, D. A., Marquette, C. H., & Cardillo, G. (2015). ERS task force statement: diagnosis and treatment of primary spontaneous pneumothorax. The European respiratory journal, 46(2), 321–335.
- Kelly, A. M., Loy, J., Tsang, A. Y., & Graham, C. A. (2006). Estimating the rate of re-expansion of spontaneous pneumothorax by a formula derived from computed tomography volumetry studies. Emergency medicine journal : EMJ, 23(10), 780–782.
- Collins, C. D., Lopez, A., Mathie, A., Wood, V., Jackson, J. E., & Roddie, M. E. (1995). Quantification of pneumothorax size on chest radiographs using interpleural distances:regression analysis based on volume measurements from helical CT. AJR. American journal of roentgenology, 165(5), 1127–1130.
- Parlak, M., Uil, S. M., & van den Berg, J. W. (2012). A prospective, randomised trial of pneumothorax therapy: manual aspiration versus conventional chest tube. Respiratory medicine, 106(11), 1600–1605.
- Pasquier M, Hugli O, Carron PN. Videos in clinical medicine. Needle aspiration of primary spontaneous pneumothorax. N Engl J Med. 2013 May 9;368(19):e24.
- Zehtabchi, S., & Rios, C. L. (2008). Management of emergency department patients with primary spontaneous pneumothorax: needle aspiration or tube thoracostomy? Annals of emergency medicine, 51(1), 91–100.e1.
- Thelle, A., Gjerdevik, M., SueChu, M., Hagen, O. M., & Bakke, P. (2017). Randomised comparison of needle aspiration and chest tube drainage in spontaneous pneumothorax. The European respiratory journal, 49(4), 1601296.
- Brown, S., Ball, E. L., Perrin, K., Asha, S. E., Braithwaite, I., Egerton-Warburton, D., Jones, P.G., Keijzers, G., Kinnear, F. B., Kwan, B., Lam, K. V., Lee, Y., Nowitz, M., Read, C. A., Simpson,G., Smith, J. A., Summers, Q. A., Weatherall, M., Beasley, R., & PSP Investigators (2020). Conservative versus Interventional Treatment for Spontaneous Pneumothorax. The New England journal of medicine, 382(5), 405–415.
- Park, C. B., Moon, M. H., Jeon, H. W., Cho, D. G., Song, S. W., Won, Y. D., Kim, Y. H., Kim, Y., Jeong, S. C., Kim, K. S., & Choi, S. Y. (2017). Does oxygen therapy increase the resolution rate of primary spontaneous pneumothorax? Journal of Thoracic Disease, 9(12), 5239-5243.
- Hallifax, R. J., McKeown, E., Sivakumar, P., Fairbairn, I., Peter, C., Leitch, A., Knight, M.,Stanton, A., et al.(2020). Ambulatory management of primary spontaneous pneumothorax: an open-label, randomised controlled trial. Lancet (London, England), 396(10243), 39–49.
- Morgenstern, J. (2020, March 21). Conservative treatment for primary spontaneous pneumothorax. Retrieved November 1, 2020, from https://first10em.com/conservative- treatment-for-pneumothorax/
Drs. Helman, Tahiri and Yehudaiff have no conflicts of interest to declare
Now test your knowledge with a quiz.





Great and timely discussion thank you. With regards the referral to thoracic surgeon. Does the ED use the dystrophy severity score on high res CT chest as part of the referral process or do they leave that to the thoracic surgeon?
In Canada I’ve never seen CT ordered in ED specifically for spontaneous pneumothorax by EM. If there is a complication that would require a CT, throacics/surgery would be involved early and may request a CT at that time, although very rarely. Unfortunately there are no patient characteristics that predict recurrence well https://www.hindawi.com/journals/crj/2017/2729548/ so I see why the dystrophy severity score looks appealing. However, the score is based on a study of only 56 patients. I don’t think we should adapt that score until much larger prospective RCTs are done and validated (the authors of the study agree).
Could you please add activities that you should avoid and for how long in the patient handout? ie Diving, Flying etc.
Also here’s a scenario: chest trauma patient with no obvious displaced rib fracture or pneumothorax. But clinically you suspect a fracture. He says he has a 10h flight back home tomorrow or even in 3 days time. Do we need to do a CT Chest to confirm if there is no small pneumothorax or its not clinically significant to have a small CXR undetectable pneumothorax?
Hello Mohan
I just read your comment. To answer at least partly, for flying after a pneumothorax there are quite clear guidelines by IATA.org (the manual can be ordered free of charge here: https://www.iata.org/en/publications/medical-manual/).
Another document that I find useful when assessing patient’s fit-to-fly status (after a pneumothorax, but also many other conditions) is the following: https://aeromedicalguidelines.com/kapitel8-gb.html
@Anton: thanks for your fantastic work!
Best regards
Martin
Greetings Dr. Helman,
Thank you so much for what you do. I recently had a case of nontraumatic large pneumothorax in a 40 year old. I was looking to learn more and came to your site. I think you provide something that is somewhat lacking in the portion of the FOAMed universe that I frequent: in depth high-quality discussion about a specific topic. As I transition from residency to attending I think your site is perfect. Sincerely thank you.
-Kurt